Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes
- PMID: 24965589
- PMCID: PMC4216938
- DOI: 10.1152/ajpcell.00049.2014
Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes
Abstract
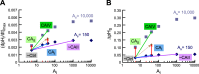
Exposing an oocyte to CO2/HCO3 (-) causes intracellular pH (pHi) to decline and extracellular-surface pH (pHS) to rise to a peak and decay. The two companion papers showed that oocytes injected with cytosolic carbonic anhydrase II (CA II) or expressing surface CA IV exhibit increased maximal rate of pHi change (dpHi/dt)max, increased maximal pHS changes (ΔpHS), and decreased time constants for pHi decline and pHS decay. Here we investigate these results using refinements of an earlier mathematical model of CO2 influx into a spherical cell. Refinements include 1) reduced cytosolic water content, 2) reduced cytosolic diffusion constants, 3) refined CA II activity, 4) layer of intracellular vesicles, 5) reduced membrane CO2 permeability, 6) microvilli, 7) refined CA IV activity, 8) a vitelline membrane, and 9) a new simulation protocol for delivering and removing the bulk extracellular CO2/HCO3 (-) solution. We show how these features affect the simulated pHi and pHS transients and use the refined model with the experimental data for 1.5% CO2/10 mM HCO3 (-) (pHo = 7.5) to find parameter values that approximate ΔpHS, the time to peak pHS, the time delay to the start of the pHi change, (dpHi/dt)max, and the change in steady-state pHi. We validate the revised model against data collected as we vary levels of CO2/HCO3 (-) or of extracellular HEPES buffer. The model confirms the hypothesis that CA II and CA IV enhance transmembrane CO2 fluxes by maximizing CO2 gradients across the plasma membrane, and it predicts that the pH effects of simultaneously implementing intracellular and extracellular-surface CA are supra-additive.
Keywords: buffers; competing equilibria; intracellular pH; reaction-diffusion; surface pH; tortuosity factors.
Copyright © 2014 the American Physiological Society.
Figures
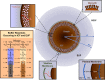
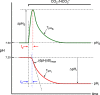
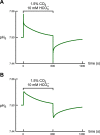
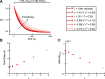
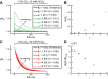
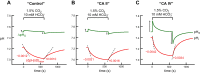


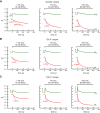

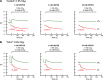
Comment in
-
How carbonic anhydrases and pH buffers facilitate the movement of carbon dioxide through biological membranes. Focus on "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes"; "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes"; and "Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes".Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C788-90. doi: 10.1152/ajpcell.00198.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965588 No abstract available.
Similar articles
-
Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes.Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C814-40. doi: 10.1152/ajpcell.00050.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965590 Free PMC article.
-
Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes.Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C791-813. doi: 10.1152/ajpcell.00051.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965587 Free PMC article.
-
How carbonic anhydrases and pH buffers facilitate the movement of carbon dioxide through biological membranes. Focus on "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes"; "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes"; and "Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes".Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C788-90. doi: 10.1152/ajpcell.00198.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965588 No abstract available.
-
Carotid body chemoreception: the importance of CO2-HCO3- and carbonic anhydrase. (review).Biol Res. 1993;26(3):319-29. Biol Res. 1993. PMID: 7606251 Review.
-
Mathematical modeling of acid-base physiology.Prog Biophys Mol Biol. 2015 Jan;117(1):43-58. doi: 10.1016/j.pbiomolbio.2015.01.003. Epub 2015 Jan 22. Prog Biophys Mol Biol. 2015. PMID: 25617697 Free PMC article. Review.
Cited by
-
Computational model of electrode-induced microenvironmental effects on pH measurements near a cell membrane.Multiscale Model Simul. 2020;18(2):1053-1075. doi: 10.1137/19m1262875. Epub 2020 May 28. Multiscale Model Simul. 2020. PMID: 34456639 Free PMC article.
-
Mechanism of CO2 and NH3 Transport through Human Aquaporin 1: Evidence for Parallel CO2 Pathways.bioRxiv [Preprint]. 2025 Jun 23:2025.02.28.640247. doi: 10.1101/2025.02.28.640247. bioRxiv. 2025. PMID: 40093180 Free PMC article. Preprint.
-
Carbon dioxide transport across membranes.Interface Focus. 2021 Apr 6;11(2):20200090. doi: 10.1098/rsfs.2020.0090. Epub 2021 Feb 12. Interface Focus. 2021. PMID: 33633837 Free PMC article. Review.
-
Carbonic anhydrases enhance activity of endogenous Na-H exchangers and not the electrogenic Na/HCO3 cotransporter NBCe1-A, expressed in Xenopus oocytes.J Physiol. 2020 Dec;598(24):5821-5856. doi: 10.1113/JP280143. Epub 2020 Oct 11. J Physiol. 2020. PMID: 32969493 Free PMC article.
-
Computational modeling predicts ephemeral acidic microdomains in the glutamatergic synaptic cleft.Biophys J. 2021 Dec 21;120(24):5575-5591. doi: 10.1016/j.bpj.2021.11.011. Epub 2021 Nov 11. Biophys J. 2021. PMID: 34774503 Free PMC article.
References
-
- Alessandrini A, Gavazzo P, Picco C, Facci P. Voltage-induced morphological modifications in oocyte membranes containing exogenous K+ channels studied by electrochemical scanning force microscopy. Microsc Res Tech 71: 274–278, 2008 - PubMed
-
- Bevensee MO, Boron WF. Control of intracellular pH. In: Seldin and Giebisch's The Kidney: Physiology & Pathophysiology. New York: Academic, 2013, p. 1773–1835
-
- Boron W. Acid-base physiology. In: Medical Physiology, edited by Boron W, Boulpaep E. Philadelphia, PA: Saunders Elsevier, 2012, p. 652–671
-
- Boron WF, Endeward V, Gros G, Musa-Aziz R, Pohl P. Intrinsic CO2 permeability of cell membranes and potential biological relevance of CO2 channels. Chemphyschem 12: 1017–1019, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

