Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes
- PMID: 24965590
- PMCID: PMC4216941
- DOI: 10.1152/ajpcell.00050.2014
Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes
Abstract
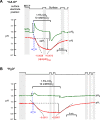
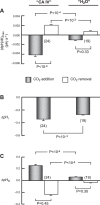
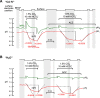
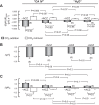
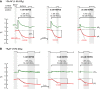
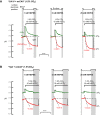
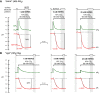
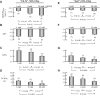
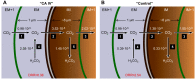
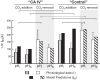
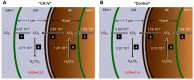
Human carbonic anhydrase IV (CA IV) is GPI-anchored to the outer membrane surface, catalyzing CO2/HCO3 (-) hydration-dehydration. We examined effects of heterologously expressed CA IV on intracellular-pH (pHi) and surface-pH (pHS) transients caused by exposing oocytes to CO2/HCO3 (-)/pH 7.50. CO2 influx causes a sustained pHi fall and a transient pHS rise; CO2 efflux does the opposite. Both during CO2 addition and removal, CA IV increases magnitudes of maximal rate of pHi change (dpHi/dt)max, and maximal pHS change (ΔpHS) and decreases time constants for pHi changes (τpHi ) and pHS relaxations (τpHS ). Decreases in time constants indicate that CA IV enhances CO2 fluxes. Extracellular acetazolamide blocks all CA IV effects, but not those of injected CA II. Injected acetazolamide partially reduces CA IV effects. Thus, extracellular CA is required for, and the equivalent of cytosol-accessible CA augments, the effects of CA IV. Increasing the concentration of the extracellular non-CO2/HCO3 (-) buffer (i.e., HEPES), in the presence of extracellular CA or at high [CO2], accelerates CO2 influx. Simultaneous measurements with two pHS electrodes, one on the oocyte meridian perpendicular to the axis of flow and one downstream from the direction of extracellular-solution flow, reveal that the downstream electrode has a larger (i.e., slower) τpHS , indicating [CO2] asymmetry over the oocyte surface. A reaction-diffusion mathematical model (third paper in series) accounts for the above general features, and supports the conclusion that extracellular CA, which replenishes entering CO2 or consumes exiting CO2 at the extracellular surface, enhances the gradient driving CO2 influx across the cell membrane.
Keywords: HEPES; acetazolamide; electrophysiology; ion-sensitive microelectrodes; mathematical modeling.
Copyright © 2014 the American Physiological Society.
Figures









Comment in
-
How carbonic anhydrases and pH buffers facilitate the movement of carbon dioxide through biological membranes. Focus on "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes"; "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes"; and "Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes".Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C788-90. doi: 10.1152/ajpcell.00198.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965588 No abstract available.
Similar articles
-
Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes.Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C791-813. doi: 10.1152/ajpcell.00051.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965587 Free PMC article.
-
Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes.Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C841-58. doi: 10.1152/ajpcell.00049.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965589 Free PMC article.
-
How carbonic anhydrases and pH buffers facilitate the movement of carbon dioxide through biological membranes. Focus on "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes"; "Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes"; and "Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes".Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C788-90. doi: 10.1152/ajpcell.00198.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965588 No abstract available.
-
Carotid body chemoreception: the importance of CO2-HCO3- and carbonic anhydrase. (review).Biol Res. 1993;26(3):319-29. Biol Res. 1993. PMID: 7606251 Review.
-
Gas transfer in dogfish: a unique model of CO2 excretion.Comp Biochem Physiol A Mol Integr Physiol. 2010 Apr;155(4):476-85. doi: 10.1016/j.cbpa.2009.10.043. Epub 2009 Nov 5. Comp Biochem Physiol A Mol Integr Physiol. 2010. PMID: 19896550 Review.
Cited by
-
Potential Novel Role of Membrane-Associated Carbonic Anhydrases in the Kidney.Int J Mol Sci. 2023 Feb 20;24(4):4251. doi: 10.3390/ijms24044251. Int J Mol Sci. 2023. PMID: 36835660 Free PMC article. Review.
-
Computational model of electrode-induced microenvironmental effects on pH measurements near a cell membrane.Multiscale Model Simul. 2020;18(2):1053-1075. doi: 10.1137/19m1262875. Epub 2020 May 28. Multiscale Model Simul. 2020. PMID: 34456639 Free PMC article.
-
Genetic disruption of the pHi-regulating proteins Na+/H+ exchanger 1 (SLC9A1) and carbonic anhydrase 9 severely reduces growth of colon cancer cells.Oncotarget. 2017 Feb 7;8(6):10225-10237. doi: 10.18632/oncotarget.14379. Oncotarget. 2017. PMID: 28055960 Free PMC article.
-
Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase II enhances CO2 fluxes across Xenopus oocyte plasma membranes.Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C791-813. doi: 10.1152/ajpcell.00051.2014. Epub 2014 Jun 25. Am J Physiol Cell Physiol. 2014. PMID: 24965587 Free PMC article.
-
Carbon dioxide transport across membranes.Interface Focus. 2021 Apr 6;11(2):20200090. doi: 10.1098/rsfs.2020.0090. Epub 2021 Feb 12. Interface Focus. 2021. PMID: 33633837 Free PMC article. Review.
References
-
- Becker HM, Deitmer JW. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3− cotransporter. J Biol Chem 282: 13508–13521, 2007 - PubMed
-
- Busa WB, Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol Regul Integr Comp Physiol 246: R409–R438, 1984 - PubMed
-
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci 15: 396–402, 1992 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

