Solid oral forms availability in children: a cost saving investigation
- PMID: 24965935
- PMCID: PMC4243883
- DOI: 10.1111/bcp.12442
Solid oral forms availability in children: a cost saving investigation
Abstract
Aim: To assess the suitability and potential cost savings, from both the hospital and community perspective, of prescribed oral liquid medicine substitution with acceptable solid forms for children over 2 years.
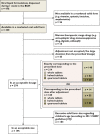
Method: Oral liquid medicines dispensed from a paediatric hospital (UK) in 1 week were assessed by screening for existence of the solid form alternative and evaluating the acceptability of the available solid form, firstly related to the prescribed dose and secondly to acceptable size depending on the child's age. Costs were calculated based on providing treatment for 28 days or prescribed duration for short term treatments.
Results: Over 90% (440/476) of liquid formulations were available as a marketed solid form. Considering dosage acceptability (maximum of 10% deviation from prescribed dosage or 0% for narrow therapeutic range drugs, maximum tablet divisions into quarters) 80% of liquids could be substituted with a solid form. The main limitation for liquid substitution would be solid form size. However, two-thirds of prescribed liquids could have been substituted with a suitable solid form for dosage and size, with estimated savings being of £5K and £8K in 1 week, respectively based on hospital and community costs, corresponding to a projected annual saving of £238K and £410K (single institution).
Conclusion: Whilst not all children over 2 years will be able to swallow tablets, drug cost savings if oral liquid formulations were substituted with suitable solid dosage forms would be considerable. Given the numerous advantages of solid forms compared with liquids, this study may provide a theoretical basis for investing in supporting children to swallow tablets/capsules.
Keywords: drug formulation; oral drug delivery; paediatric; swallowing.
© 2014 The British Pharmacological Society.
Figures
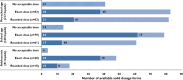
 , no acceptable dose;
, no acceptable dose;  , acceptable dose for whole tablet;
, acceptable dose for whole tablet;  , acceptable dose after splitting tablet)
, acceptable dose after splitting tablet)References
-
- Salunke S, Hempenstall J, Kendall R, Roger B, Mroz C, Nunn T, Tuleu C. European Paediatric Formulation Initiative's (EuPFI) 2nd conference commentary – Formulating better medicines for children. Int J Pharm. 2011;419:235–239. - PubMed
-
- European Medicines Agency, Committee for Medicinal Products for Human Use. 2006. Reflection paper: formulations of choice for the paediatric population; EMEA/CHMP/PEG/194810/2005.
-
- Walsh J, Mills S. Conference report: formulating better medicines for children: 4th European Paediatric Formulation Initiative conference. Ther Deliv. 2013;4:21–25. - PubMed
-
- Cram A, Breitkreutz J, Desset-Brethes S, Nunn T, Tuleu C European Paediatric Formulation I. Challenges of developing palatable oral paediatric formulations. Int J Pharm. 2009;365:1–3. - PubMed
-
- Nahata MC. Lack of pediatric drug formulations. Pediatrics. 1999;104:607–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

