Temperature systematically modifies neural activity for sweet taste
- PMID: 24966301
- PMCID: PMC4157175
- DOI: 10.1152/jn.00368.2014
Temperature systematically modifies neural activity for sweet taste
Abstract
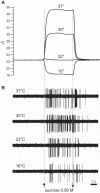
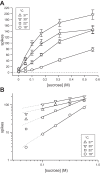
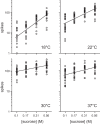
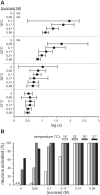
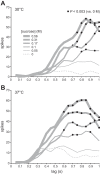
Temperature can modify neural and behavioral responses to taste stimuli that elicit "sweetness," a perception linked to intake of calorie-laden foods. However, the role of temperature in the neural representation of sweet taste is poorly understood. Here we made electrophysiological recordings from gustatory neurons in the medulla of inbred mice to study how adjustments in taste solution temperature to cool (18°C), ambient (22°C), and warm (30°C and 37°C) values changed the magnitude and latency of gustatory activity to sucrose (0, 0.05, 0.1, 0.17, 0.31, and 0.56 M). Analysis of 22 sucrose-best neurons revealed that temperature markedly influenced responses to sucrose, which, across concentrations, were largest when solutions were warmed to 30°C. However, reducing solution temperature from warm to ambient to cool progressively steepened the slope of the sucrose concentration-response function computed across cells (P < 0.05), indicating that mean activity to sucrose increased more rapidly with concentration steps under cooling than with warming. Thus the slope of the sucrose concentration-response function shows an inverse relation with temperature. Temperature also influenced latency to the first spike of the sucrose response. Across neurons, latencies were shorter when sucrose solutions were warmed and longer, by hundreds of milliseconds, when solutions were cooled (P < 0.05), indicating that temperature is also a temporal parameter of sucrose activity. Our findings reveal that temperature systematically modifies the timing of gustatory activity to sucrose in the mammalian brain and how this activity changes with concentration. Results further highlight how oral somatosensory cues function as physiological modulators of gustatory processing.
Keywords: coding; latency; sucrose; taste; temperature.
Copyright © 2014 the American Physiological Society.
Figures

References
-
- Bair W, Koch C. Temporal precision of spike trains in extrastriate cortex of the behaving macaque monkey. Neural Comput 8: 1185–1202, 1996 - PubMed
-
- Bartoshuk LM, Rennert K, Rodin J, Stevens JC. Effects of temperature on the perceived sweetness of sucrose. Physiol Behav 28: 905–910, 1982 - PubMed
-
- Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol 95: 674–685, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

