Antiviral responses in mouse embryonic stem cells: differential development of cellular mechanisms in type I interferon production and response
- PMID: 24966329
- PMCID: PMC4155682
- DOI: 10.1074/jbc.M113.537746
Antiviral responses in mouse embryonic stem cells: differential development of cellular mechanisms in type I interferon production and response
Abstract
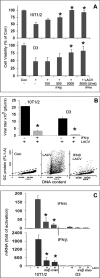
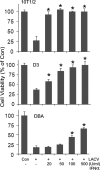
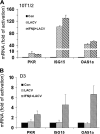
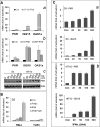
We have recently reported that mouse embryonic stem cells (mESCs) are deficient in expressing type I interferons (IFNs) in response to viral infection and synthetic viral RNA analogs (Wang, R., Wang, J., Paul, A. M., Acharya, D., Bai, F., Huang, F., and Guo, Y. L. (2013) J. Biol. Chem. 288, 15926-15936). Here, we report that mESCs are able to respond to type I IFNs, express IFN-stimulated genes, and mediate the antiviral effect of type I IFNs against La Crosse virus and chikungunya virus. The major signaling components in the IFN pathway are expressed in mESCs. Therefore, the basic molecular mechanisms that mediate the effects of type I IFNs are functional in mESCs; however, these mechanisms may not yet be fully developed as mESCs express lower levels of IFN-stimulated genes and display weaker antiviral activity in response to type I IFNs when compared with fibroblasts. Further analysis demonstrated that type I IFNs do not affect the stem cell state of mESCs. We conclude that mESCs are deficient in type I IFN expression, but they can respond to and mediate the cellular effects of type I IFNs. These findings represent unique and uncharacterized properties of mESCs and are important for understanding innate immunity development and ESC physiology.
Keywords: Antiviral Response; Double-stranded RNA (dsRNA); Embryonic Stem Cell; Innate Immunity; Interferon; Interferon-stimulated Genes; Viral Replication.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Development of Antiviral Innate Immunity During In Vitro Differentiation of Mouse Embryonic Stem Cells.Stem Cells Dev. 2016 Apr 15;25(8):648-59. doi: 10.1089/scd.2015.0377. Epub 2016 Mar 17. Stem Cells Dev. 2016. PMID: 26906411 Free PMC article.
-
Mouse embryonic stem cells are deficient in type I interferon expression in response to viral infections and double-stranded RNA.J Biol Chem. 2013 May 31;288(22):15926-36. doi: 10.1074/jbc.M112.421438. Epub 2013 Apr 11. J Biol Chem. 2013. PMID: 23580653 Free PMC article.
-
Induction of USP25 by viral infection promotes innate antiviral responses by mediating the stabilization of TRAF3 and TRAF6.Proc Natl Acad Sci U S A. 2015 Sep 8;112(36):11324-9. doi: 10.1073/pnas.1509968112. Epub 2015 Aug 24. Proc Natl Acad Sci U S A. 2015. PMID: 26305951 Free PMC article.
-
The antiviral activities of ISG15.J Mol Biol. 2013 Dec 13;425(24):4995-5008. doi: 10.1016/j.jmb.2013.09.041. Epub 2013 Oct 3. J Mol Biol. 2013. PMID: 24095857 Free PMC article. Review.
-
Interfering with immunity: detrimental role of type I IFNs during infection.J Immunol. 2015 Mar 15;194(6):2455-65. doi: 10.4049/jimmunol.1402794. J Immunol. 2015. PMID: 25747907 Review.
Cited by
-
Engineered Mammalian RNAi Can Elicit Antiviral Protection that Negates the Requirement for the Interferon Response.Cell Rep. 2015 Nov 17;13(7):1456-1466. doi: 10.1016/j.celrep.2015.10.020. Epub 2015 Nov 5. Cell Rep. 2015. PMID: 26549455 Free PMC article.
-
Establishment, characterization, and validation of novel porcine embryonic fibroblasts as a potential source for genetic modification.Front Cell Dev Biol. 2022 Nov 10;10:1059710. doi: 10.3389/fcell.2022.1059710. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36438568 Free PMC article.
-
No evidence for viral small RNA production and antiviral function of Argonaute 2 in human cells.Sci Rep. 2019 Sep 24;9(1):13752. doi: 10.1038/s41598-019-50287-w. Sci Rep. 2019. PMID: 31551491 Free PMC article.
-
Dicer and PKR as Novel Regulators of Embryonic Stem Cell Fate and Antiviral Innate Immunity.J Immunol. 2022 May 15;208(10):2259-2266. doi: 10.4049/jimmunol.2200042. J Immunol. 2022. PMID: 35577384 Free PMC article. Review.
-
Antiviral RNA silencing in mammals: no news is not good news.Cell Rep. 2014 Nov 6;9(3):795-7. doi: 10.1016/j.celrep.2014.10.029. Cell Rep. 2014. PMID: 25437534 Free PMC article. No abstract available.
References
-
- Niwa H. (2007) How is pluripotency determined and maintained? Development 134, 635–646 - PubMed
-
- Wobus A. M., Boheler K. R. (2005) Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol. Rev. 85, 635–678 - PubMed
-
- Zampetaki A., Xiao Q., Zeng L., Hu Y., Xu Q. (2006) TLR4 expression in mouse embryonic stem cells and in stem cell-derived vascular cells is regulated by epigenetic modifications. Biochem. Biophys. Res. Commun. 347, 89–99 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

