Roles of ON cone bipolar cell subtypes in temporal coding in the mouse retina
- PMID: 24966376
- PMCID: PMC4069354
- DOI: 10.1523/JNEUROSCI.3965-13.2014
Roles of ON cone bipolar cell subtypes in temporal coding in the mouse retina
Abstract
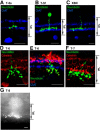
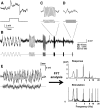
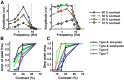
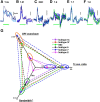
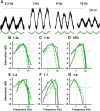
In the visual system, diverse image processing starts with bipolar cells, which are the second-order neurons of the retina. Thirteen subtypes of bipolar cells have been identified, which are thought to encode different features of image signaling and to initiate distinct signal-processing streams. Although morphologically identified, the functional roles of each bipolar cell subtype in visual signal encoding are not fully understood. Here, we investigated how ON cone bipolar cells of the mouse retina encode diverse temporal image signaling. We recorded bipolar cell voltage changes in response to two different input functions: sinusoidal light and step light stimuli. Temporal tuning in ON cone bipolar cells was diverse and occurred in a subtype-dependent manner. Subtypes 5s and 8 exhibited low-pass filtering property in response to a sinusoidal light stimulus, and responded with sustained fashion to step-light stimulation. Conversely, subtypes 5f, 6, 7, and XBC exhibited bandpass filtering property in response to sinusoidal light stimuli, and responded transiently to step-light stimuli. In particular, subtypes 7 and XBC were high-temporal tuning cells. We recorded responses in different ways to further examine the underlying mechanisms of temporal tuning. Current injection evoked low-pass filtering, whereas light responses in voltage-clamp mode produced bandpass filtering in all ON bipolar cells. These findings suggest that cone photoreceptor inputs shape bandpass filtering in bipolar cells, whereas intrinsic properties of bipolar cells shape low-pass filtering. Together, our results demonstrate that ON bipolar cells encode diverse temporal image signaling in a subtype-dependent manner to initiate temporal visual information-processing pathways.
Keywords: light response; parallel processing; patch-clamp; sine wave; subtypes; voltage-gated channels.
Copyright © 2014 the authors 0270-6474/14/348761-11$15.00/0.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
