Porcine reproductive and respiratory syndrome virus induces IL-1β production depending on TLR4/MyD88 pathway and NLRP3 inflammasome in primary porcine alveolar macrophages
- PMID: 24966466
- PMCID: PMC4055429
- DOI: 10.1155/2014/403515
Porcine reproductive and respiratory syndrome virus induces IL-1β production depending on TLR4/MyD88 pathway and NLRP3 inflammasome in primary porcine alveolar macrophages
Abstract
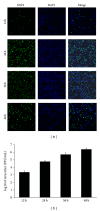
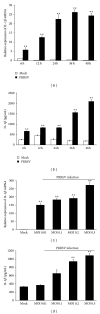
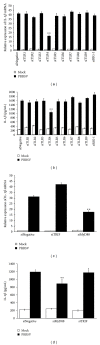
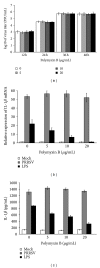
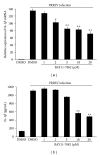
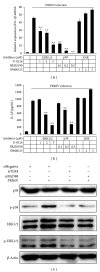
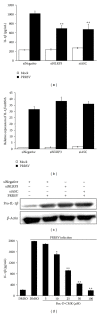
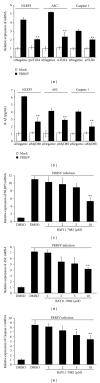
Porcine reproductive and respiratory syndrome virus (PRRSV) is an Arterivirus that has been devastating the swine industry worldwide since the late 1980s. Previous studies have reported that PRRSV infection induced the production of IL-1 β . However, the cellular sensors and signaling pathways involved in this process have not been elucidated yet. Here, we studied the mechanisms responsible for the production of IL-1 β in response to highly pathogenic PRRSV. Upon PRRSV infection of primary porcine alveolar macrophages, both mRNA expression and secretion of IL-1 β were significantly increased in a time- and dose-dependent manner. We also investigated the role of several pattern-recognition receptors and adaptor molecules in this response and showed that the TLR4/MyD88 pathway and its downstream signaling molecules, NF- κ B, ERK1/2, and p38 MAPKs, were involved in IL-1 β production during PRRSV infection. Treatment with specific inhibitors or siRNA knockdown assays demonstrated that components of the NLRP3 inflammasome were crucial for IL-1 β secretion but not for IL-1 β mRNA expression. Furthermore, TLR4/MyD88/NF- κ B signaling pathway was involved in PRRSV-induced expression of NLRP3 inflammasome components. Together, our results deciphered the pathways leading from recognition of PRRSV to the production and release of IL-1 β , providing a deeper knowledge of the mechanisms of PRRSV-induced inflammation responses.
Figures
Similar articles
-
IL-1β induced by PRRSV co-infection inhibited CSFV C-strain proliferation via the TLR4/NF-κB/MAPK pathways and the NLRP3 inflammasome.Vet Microbiol. 2022 Oct;273:109513. doi: 10.1016/j.vetmic.2022.109513. Epub 2022 Jul 15. Vet Microbiol. 2022. PMID: 35952491
-
Porcine reproductive and respiratory syndrome virus infection activates IL-10 production through NF-κB and p38 MAPK pathways in porcine alveolar macrophages.Dev Comp Immunol. 2013 Mar;39(3):265-72. doi: 10.1016/j.dci.2012.10.001. Epub 2012 Oct 22. Dev Comp Immunol. 2013. PMID: 23085400
-
Porcine reproductive and respiratory syndrome virus induces interleukin-1β through MyD88/ERK/AP-1 and NLRP3 inflammasome in microglia.Vet Microbiol. 2018 Dec;227:82-89. doi: 10.1016/j.vetmic.2018.10.030. Epub 2018 Oct 30. Vet Microbiol. 2018. PMID: 30473357
-
Antagonizing interferon-mediated immune response by porcine reproductive and respiratory syndrome virus.Biomed Res Int. 2014;2014:315470. doi: 10.1155/2014/315470. Epub 2014 Jul 3. Biomed Res Int. 2014. PMID: 25101271 Free PMC article. Review.
-
Exploring the Use of Medicinal Plants and Their Bioactive Derivatives as Alveolar NLRP3 Inflammasome Regulators during Mycobacterium tuberculosis Infection.Int J Mol Sci. 2021 Aug 31;22(17):9497. doi: 10.3390/ijms22179497. Int J Mol Sci. 2021. PMID: 34502407 Free PMC article. Review.
Cited by
-
Antiviral Strategies of Chinese Herbal Medicine Against PRRSV Infection.Front Microbiol. 2020 Jul 28;11:1756. doi: 10.3389/fmicb.2020.01756. eCollection 2020. Front Microbiol. 2020. PMID: 32849384 Free PMC article. Review.
-
Inflammasome control of viral infection.Curr Opin Virol. 2015 Jun;12:38-46. doi: 10.1016/j.coviro.2015.02.007. Epub 2015 Mar 12. Curr Opin Virol. 2015. PMID: 25771504 Free PMC article. Review.
-
Peste Des Petits Ruminants Virus N Protein Is a Critical Proinflammation Factor That Promotes MyD88 and NLRP3 Complex Assembly.J Virol. 2022 May 25;96(10):e0030922. doi: 10.1128/jvi.00309-22. Epub 2022 May 3. J Virol. 2022. PMID: 35502911 Free PMC article.
-
The Antiviral Effect of Isatis Root Polysaccharide against NADC30-like PRRSV by Transcriptome and Proteome Analysis.Int J Mol Sci. 2022 Mar 28;23(7):3688. doi: 10.3390/ijms23073688. Int J Mol Sci. 2022. PMID: 35409050 Free PMC article.
-
Damage to intestinal barrier integrity in piglets caused by porcine reproductive and respiratory syndrome virus infection.Vet Res. 2021 Jun 23;52(1):93. doi: 10.1186/s13567-021-00965-3. Vet Res. 2021. PMID: 34162433 Free PMC article.
References
-
- Bose S, Banerjee AK. Innate immune response against nonsegmented negative strand RNA viruses. Journal of Interferon and Cytokine Research. 2003;23(8):401–412. - PubMed
-
- Dinarello CA. Biologic basis for Interleukin-1 in disease. Blood. 1996;87(6):2095–2147. - PubMed
-
- Eder C. Mechanisms of interleukin-1β release. Immunobiology. 2009;214(7):543–553. - PubMed
-
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

