Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update
- PMID: 24966597
- PMCID: PMC4064072
- DOI: 10.3748/wjg.v20.i23.7260
Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update
Abstract
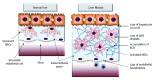
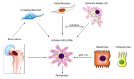
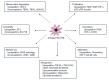
There have been considerable recent advances towards a better understanding of the complex cellular and molecular network underlying liver fibrogenesis. Recent data indicate that the termination of fibrogenic processes and the restoration of deficient fibrolytic pathways may allow the reversal of advanced fibrosis and even cirrhosis. Therefore, efforts have been made to better clarify the cellular and molecular mechanisms that are involved in liver fibrosis. Activation of hepatic stellate cells (HSCs) remains a central event in fibrosis, complemented by other sources of matrix-producing cells, including portal fibroblasts, fibrocytes and bone marrow-derived myofibroblasts. These cells converge in a complex interaction with neighboring cells to provoke scarring in response to persistent injury. Defining the interaction of different cell types, revealing the effects of cytokines on these cells and characterizing the regulatory mechanisms that control gene expression in activated HSCs will enable the discovery of new therapeutic targets. Moreover, the characterization of different pathways associated with different etiologies aid in the development of disease-specific therapies. This article outlines recent advances regarding the cellular and molecular mechanisms involved in liver fibrosis that may be translated into future therapies. The pathogenesis of liver fibrosis associated with alcoholic liver disease, non-alcoholic fatty liver disease and viral hepatitis are also discussed to emphasize the various mechanisms involved in liver fibrosis.
Keywords: Cirrhosis; Extracellular matrix; Fibrogenesis; Hepatic stellate cells; Liver; Liver fibrosis; Myofibroblast.
Figures
References
-
- Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473–1492. - PubMed
-
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. - PubMed
-
- Rockey DC. Antifibrotic therapy in chronic liver disease. Clin Gastroenterol Hepatol. 2005;3:95–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

