Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies
- PMID: 24966672
- PMCID: PMC4064950
- DOI: 10.2147/COPD.S61717
Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies
Abstract
Background: Olodaterol is a long-acting β2-agonist with a 24-hour bronchodilator profile. Two replicate, randomized, double-blind, placebo-controlled, parallel-group, Phase III trials were performed as part of a comprehensive clinical program to investigate the long-term safety and efficacy of olodaterol in patients with moderate to very severe chronic obstructive pulmonary disease (COPD) receiving usual-care background therapy.
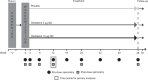
Methods: Patients received olodaterol 5 μg or 10 μg or placebo once daily for 48 weeks. Coprimary end points were forced expiratory volume in 1 second (FEV1) area under the curve from 0 to 3 hours (AUC0-3) response (change from baseline), and trough FEV1 response at 12 weeks. Secondary end points included additional lung function assessments, use of rescue medications, FEV1 AUC response from 0 to 12 hours, and Patient Global Rating over 48 weeks.
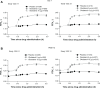
Results: Overall, 624 and 642 patients were evaluated in studies 1222.11 and 1222.12, respectively. In both studies, olodaterol 5 μg and 10 μg significantly improved the FEV1 AUC0-3 response (P<0.0001) and trough FEV1 (study 1222.11, P<0.0001; study 1222.12, P<0.05, post hoc) at week 12, with an incidence of adverse events comparable with that of placebo. Secondary end points supported the efficacy of olodaterol.
Conclusion: These studies demonstrate the long-term efficacy and safety of once-daily olodaterol 5 μg and 10 μg in patients with moderate to very severe COPD continuing with usual-care maintenance therapy.
Trial registration: ClinicalTrials.gov NCT00782210 NCT00782509.
Keywords: bronchodilator; chronic obstructive pulmonary disease; olodaterol.
Figures

References
-
- Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. [Accessed July 15, 2013]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. - PubMed
-
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. - PubMed
-
- Dahl R, Greefhorst LAPM, Nowak D, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):778–784. - PubMed
-
- Cazzola M, Santangelo G, Piccolo A, et al. Effect of salmeterol and formoterol in patients with chronic obstructive pulmonary disease. Pulm Pharmacol. 1994;7(2):103–107. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

