TSLP induced by estrogen stimulates secretion of MCP-1 and IL-8 and growth of human endometrial stromal cells through JNK and NF-κB signal pathways
- PMID: 24966899
- PMCID: PMC4069968
TSLP induced by estrogen stimulates secretion of MCP-1 and IL-8 and growth of human endometrial stromal cells through JNK and NF-κB signal pathways
Abstract
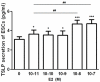
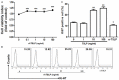
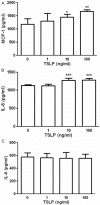
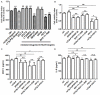
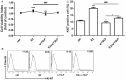
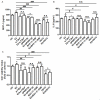
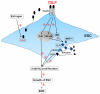
It has reported that human endometrial stromal cells (ESCs) express thymic stromal lymphopoietin (TSLP), and TSLP concentrations in the serum and peritoneal fluid were higher in women with endometriosis. Endometriosis is an estrogen-dependent disease. The present study aimed to elucidate whether and how estrogen regulates the growth of ESCs through TSLP. The ESCs behaviors in vitro were verified by SRB assay and Ki67 level detection, respectively. In addition, the effects of estrogen on TSLP and TSLP on the correspondent functional molecules were investigated by ELISA and flow cytometry. Here we found that estrogen stimulated the secretion of TSLP in a dosage-dependent manner. Recombinant human TSLP stimulates the secretion of MCP-1 and IL-8, and markedly promotes the viability and proliferation relative gene Ki-67 expression of ESCs. These effects could be abolished by the inhibitor for JNK or NF-κB signal, respectively. Moreover, not only anti-TSLP neutralizing antibody, but also blocking JNK or NF-κB signal by inhibitor abrogated the stimulatory role in the production of MCP-1 and IL-8, and the growth of ESCs induced by estrogen. Our current study has demonstrated that TSLP is involved in the regulation of estrogen on the secretion of MCP-1 and IL-8, and the growth of ESCs through JNK and NF-κB signal pathways, which suggests that the abnormal high expression of TSLP induced by estrogen may play an important role in ESCs growth and finally contribute to the origin and development of endometriosis.
Keywords: ESCs; IL-8; MCP-1; TSLP; endometriosis; estrogen; proliferation.
Figures
References
-
- Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. - PubMed
-
- Baldi A, Campioni M, Signorile PG. Endometriosis: pathogenesis, diagnosis, therapy and association with cancer (review) Oncol Rep. 2008;19:843–846. - PubMed
-
- Ulukus M, Cakmak H, Arici A. The role of endometrium in endometriosis. J Soc Gynecol Investing. 2006;13:467–476. - PubMed
-
- Barrier BF. Immunology of endometriosis. Clin Obstet Gynecol. 2010;53:397–402. - PubMed
-
- Rizner TL. Estrogen metabolism and action in endometriosis. Mol Cell Endocrinol. 2009;307:8–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
