Structure of the gut microbiome following colonization with human feces determines colonic tumor burden
- PMID: 24967088
- PMCID: PMC4070349
- DOI: 10.1186/2049-2618-2-20
Structure of the gut microbiome following colonization with human feces determines colonic tumor burden
Abstract
Background: A growing body of evidence indicates that the gut microbiome plays a role in the development of colorectal cancer (CRC). Patients with CRC harbor gut microbiomes that are structurally distinct from those of healthy individuals; however, without the ability to track individuals during disease progression, it has not been possible to observe changes in the microbiome over the course of tumorigenesis. Mouse models have demonstrated that these changes can further promote colonic tumorigenesis. However, these models have relied upon mouse-adapted bacterial populations and so it remains unclear which human-adapted bacterial populations are responsible for modulating tumorigenesis.
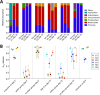
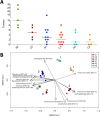
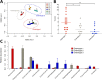
Results: We transplanted fecal microbiota from three CRC patients and three healthy individuals into germ-free mice, resulting in six structurally distinct microbial communities. Subjecting these mice to a chemically induced model of CRC resulted in different levels of tumorigenesis between mice. Differences in the number of tumors were strongly associated with the baseline microbiome structure in mice, but not with the cancer status of the human donors. Partitioning of baseline communities into enterotypes by Dirichlet multinomial mixture modeling resulted in three enterotypes that corresponded with tumor burden. The taxa most strongly positively correlated with increased tumor burden were members of the Bacteroides, Parabacteroides, Alistipes, and Akkermansia, all of which are Gram-negative. Members of the Gram-positive Clostridiales, including multiple members of Clostridium Group XIVa, were strongly negatively correlated with tumors. Analysis of the inferred metagenome of each community revealed a negative correlation between tumor count and the potential for butyrate production, and a positive correlation between tumor count and the capacity for host glycan degradation. Despite harboring distinct gut communities, all mice underwent conserved structural changes over the course of the model. The extent of these changes was also correlated with tumor incidence.
Conclusion: Our results suggest that the initial structure of the microbiome determines susceptibility to colonic tumorigenesis. There appear to be opposing roles for certain Gram-negative (Bacteroidales and Verrucomicrobia) and Gram-positive (Clostridiales) bacteria in tumor susceptibility. Thus, the impact of community structure is potentially mediated by the balance between protective, butyrate-producing populations and inflammatory, mucin-degrading populations.
Keywords: Colorectal cancer; Community structure; Germ-free; Gut microbiome; Humanized mice; Microbiota.
Figures
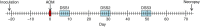

References
-
- American Cancer Society. Colorectal Cancer Facts & Figures 2011-2013. Atlanta; 2013.
-
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–215. - PMC - PubMed
-
- Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338(6103):120–123. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

