Characterizations of plasticized polymeric film coatings for preparing multiple-unit floating drug delivery systems (muFDDSs) with controlled-release characteristics
- PMID: 24967594
- PMCID: PMC4072683
- DOI: 10.1371/journal.pone.0100321
Characterizations of plasticized polymeric film coatings for preparing multiple-unit floating drug delivery systems (muFDDSs) with controlled-release characteristics
Abstract
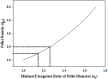
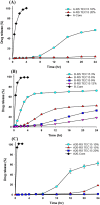
Effervescent multiple-unit floating drug delivery systems (muFDDSs) consisting of drug (lorsartan)- and effervescent (sodium bicarbonate)-containing pellets were characterized in this study. The mechanical properties (stress and strain at rupture, Young's modulus, and toughness) of these plasticized polymeric films of acrylic (Eudragit RS, RL, and NE) and cellulosic materials (ethyl cellulose (EC), and Surelease) were examined by a dynamic mechanical analyzer. Results demonstrated that polymeric films prepared from Surelease and EC were brittle with less elongation compared to acrylic films. Eudragit NE films were very flexible in both the dry and wet states. Because plasticizer leached from polymeric films during exposure to the aqueous medium, plasticization of wet Eudragit RS and RL films with 15% triethyl citrate (TEC) or diethyl phthalate (DEP) resulted in less elongation. DEP might be the plasticizer of choice among the plasticizers examined in this study for Eudragit RL to provide muFDDSs with a short time for all pellets to float (TPF) and a longer period of floating. Eudragit RL and RS at a 1∶1 ratio plasticized with 15% DEP were optimally selected as the coating membrane for the floating system. Although the release of losartan from the pellets was still too fast as a result of losartan being freely soluble in water, muFDDSs coated with Eudragit RL and RS at a 1∶1 ratio might have potential use for the sustained release of water-insoluble or the un-ionized form of drugs from gastroretentive drug delivery systems.
Conflict of interest statement
Figures


Similar articles
-
Mechanical properties of dry and wet cellulosic and acrylic films prepared from aqueous colloidal polymer dispersions used in the coating of solid dosage forms.Pharm Res. 1994 Jun;11(6):882-8. doi: 10.1023/a:1018942127524. Pharm Res. 1994. PMID: 7937530
-
Evaluation of water uptake and mechanical properties of blended polymer films for preparing gas-generated multiple-unit floating drug delivery systems.J Pharm Sci. 2012 Oct;101(10):3811-22. doi: 10.1002/jps.23279. Epub 2012 Jul 25. J Pharm Sci. 2012. PMID: 22833214
-
Floating or pulsatile drug delivery systems based on coated effervescent cores.Int J Pharm. 1999 Oct 5;187(2):175-84. doi: 10.1016/s0378-5173(99)00189-1. Int J Pharm. 1999. PMID: 10502623
-
Organic esters of plasticizers affecting the water absorption, adhesive property, glass transition temperature and plasticizer permanence of eudragit acrylic films.J Control Release. 2000 Sep 3;68(3):343-50. doi: 10.1016/s0168-3659(00)00259-5. J Control Release. 2000. PMID: 10974388
-
Preparation and in vitro evaluation of a multiple-unit floating drug delivery system based on gas formation technique.Int J Pharm. 2006 Nov 6;324(2):136-43. doi: 10.1016/j.ijpharm.2006.06.002. Epub 2006 Jun 9. Int J Pharm. 2006. PMID: 16828997
Cited by
-
Development and Optimisation of Novel Polymeric Compositions for Sustained Release Theophylline Caplets (PrintCap) via FDM 3D Printing.Polymers (Basel). 2019 Dec 21;12(1):27. doi: 10.3390/polym12010027. Polymers (Basel). 2019. PMID: 31877755 Free PMC article.
-
Expandable Drug Delivery Systems Based on Shape Memory Polymers: Impact of Film Coating on Mechanical Properties and Release and Recovery Performance.Pharmaceutics. 2022 Dec 15;14(12):2814. doi: 10.3390/pharmaceutics14122814. Pharmaceutics. 2022. PMID: 36559306 Free PMC article.
References
-
- Pawar VK, Kansal S, Garg G, Awasthi R, Singodia D, et al. (2011) Gastroretentive dosage forms: a review with special emphasis on floating drug delivery systems. Drug delivery 18: 97–110. - PubMed
-
- Waterman KC (2007) A critical review of gastric retentive controlled drug delivery. Pharmaceutical development and technology 12: 1–10. - PubMed
-
- Singh BN, Kim KH (2000) Floating drug delivery systems: An approach to oral controlled drug delivery via gastric retention. J Controlled Release 63: 235–259. - PubMed
-
- Rouge N, Buri P, Doelker E (1996) Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int J Pharm 136: 117–139.
-
- Sato Y, Kawashima Y, Takeuchi H, Yamamoto H (2004) In vitro and in vivo evaluation of riboflavin-containing microballoons for a floating controlled drug delivery system in healthy humans. Int J Pharm 275: 97–107. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

