Structural and functional photoacoustic molecular tomography aided by emerging contrast agents
- PMID: 24967718
- PMCID: PMC4569000
- DOI: 10.1039/c4cs00086b
Structural and functional photoacoustic molecular tomography aided by emerging contrast agents
Abstract
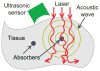
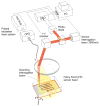
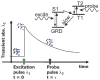
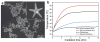
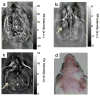
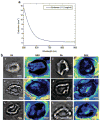
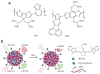
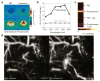
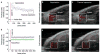
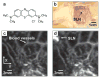
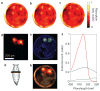
Photoacoustic tomography (PAT) can offer structural, functional and molecular contrasts at scalable observation level. By ultrasonically overcoming the strong optical scattering, this imaging technology can reach centimeters penetration depth while retaining high spatial resolution in biological tissue. Recent extensive research has been focused on developing new contrast agents to improve the imaging sensitivity, specificity and efficiency. These emerging materials have substantially accelerated PAT applications in signal sensing, functional imaging, biomarker labeling and therapy monitoring etc. Here, the potentials of different optical probes as PAT contrast agents were elucidated. We first describe the instrumental embodiments and the measured functional parameters, then focus on emerging contrast agent-based PAT applications, and finally discuss the challenges and prospects.
Figures
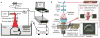








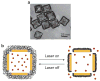

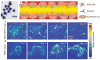
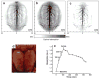

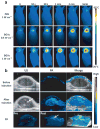

















References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

