A randomized, single-ascending-dose, ivermectin-controlled, double-blind study of moxidectin in Onchocerca volvulus infection
- PMID: 24968000
- PMCID: PMC4072596
- DOI: 10.1371/journal.pntd.0002953
A randomized, single-ascending-dose, ivermectin-controlled, double-blind study of moxidectin in Onchocerca volvulus infection
Abstract
Background: Control of onchocerciasis as a public health problem in Africa relies on annual mass ivermectin distribution. New tools are needed to achieve elimination of infection. This study determined in a small number of Onchocerca volvulus infected individuals whether moxidectin, a veterinary anthelminthic, is safe enough to administer it in a future large study to further characterize moxidectin's safety and efficacy. Effects on the parasite were also assessed.
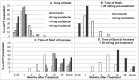
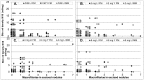
Methodology/principal findings: Men and women from a forest area in South-eastern Ghana without ivermectin mass distribution received a single oral dose of 2 mg (N = 44), 4 mg (N = 45) or 8 mg (N = 38) moxidectin or 150 µg/kg ivermectin (N = 45) with 18 months follow up. All ivermectin and 97%-100% of moxidectin treated participants had Mazzotti reactions. Statistically significantly higher percentages of participants treated with 8 mg moxidectin than participants treated with ivermectin experienced pruritus (87% vs. 56%), rash (63% vs. 42%), increased pulse rate (61% vs. 36%) and decreased mean arterial pressure upon 2 minutes standing still after ≥5 minutes supine relative to pre-treatment (61% vs. 27%). These reactions resolved without treatment. In the 8 mg moxidectin and ivermectin arms, the mean±SD number of microfilariae/mg skin were 22.9±21.1 and 21.2±16.4 pre-treatment and 0.0±0.0 and 1.1±4.2 at nadir reached 1 and 3 months after treatment, respectively. At 6 months, values were 0.0±0.0 and 1.6±4.5, at 12 months 0.4±0.9 and 3.4±4.4 and at 18 months 1.8±3.3 and 4.0±4.8, respectively, in the 8 mg moxidectin and ivermectin arm. The reduction from pre-treatment values was significantly higher after 8 mg moxidectin than after ivermectin treatment throughout follow up (p<0.01).
Conclusions/significance: The 8 mg dose of moxidectin was safe enough to initiate the large study. Provided its results confirm those from this study, availability of moxidectin to control programmes could help them achieve onchocerciasis elimination objectives.
Trial registration: ClinicalTrials.gov NCT00300768.
Conflict of interest statement
The authors have read the journal's policy and report the following matters, which could be perceived as possible sources for conflict of interests: This study was funded by the UNICEF/UNDP/World Bank/World Health Organisation Special Programme for Research and Training in Tropical Diseases (WHO/TDR) and the African Programme for Onchocerciasis Control. Pfizer provided drug for this study as well as systems for analysis of the data. This does not alter our adherence to all PLOS NTDs policies on sharing data and materials. Pfizer did not contribute to the current publication. JLH and ACK were responsible for the management of the project at WHO/TDR. They state that their employment and role in project management has not caused any conflict of interest in any of the following: study design, data collection, data analysis, data interpretation, and decision to publish.
Figures







References
-
- African Programme for Onchocerciasis Control (APOC) (2005) Final Communiqué of the 11th session of the Joint Action Forum (JAF) of APOC. WHO. Available: http://www.who.int/apoc/about/structure/jaf/jaf11_final_communique.pdf.
-
- Noma M, Nwoke BE, Nutall I, Tambala PA, Enyong P, et al. (2002) Rapid epidemiological mapping of onchocerciasis (REMO): its application by the African Programme for Onchocerciasis Control (APOC). Ann Trop Med Parasitol 96 Suppl 1: S29–S39. - PubMed
-
- Amazigo U, Boatin B (2006) The future of onchocerciasis control in Africa. Lancet 368: 1946–1947. - PubMed
-
- Awadzi K, Opoku NO, Attah SK, Addy ET, Duke BO, et al. (1997) The safety and efficacy of amocarzine in African onchocerciasis and the influence of ivermectin on the clinical and parasitological response to treatment. Ann Trop Med Parasitol 91: 281–296. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

