Cycling empirical antibiotic therapy in hospitals: meta-analysis and models
- PMID: 24968123
- PMCID: PMC4072793
- DOI: 10.1371/journal.ppat.1004225
Cycling empirical antibiotic therapy in hospitals: meta-analysis and models
Abstract
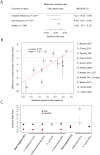
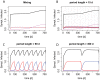
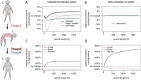
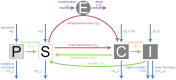
The rise of resistance together with the shortage of new broad-spectrum antibiotics underlines the urgency of optimizing the use of available drugs to minimize disease burden. Theoretical studies suggest that coordinating empirical usage of antibiotics in a hospital ward can contain the spread of resistance. However, theoretical and clinical studies came to different conclusions regarding the usefulness of rotating first-line therapy (cycling). Here, we performed a quantitative pathogen-specific meta-analysis of clinical studies comparing cycling to standard practice. We searched PubMed and Google Scholar and identified 46 clinical studies addressing the effect of cycling on nosocomial infections, of which 11 met our selection criteria. We employed a method for multivariate meta-analysis using incidence rates as endpoints and find that cycling reduced the incidence rate/1000 patient days of both total infections by 4.95 [9.43-0.48] and resistant infections by 7.2 [14.00-0.44]. This positive effect was observed in most pathogens despite a large variance between individual species. Our findings remain robust in uni- and multivariate metaregressions. We used theoretical models that reflect various infections and hospital settings to compare cycling to random assignment to different drugs (mixing). We make the realistic assumption that therapy is changed when first line treatment is ineffective, which we call "adjustable cycling/mixing". In concordance with earlier theoretical studies, we find that in strict regimens, cycling is detrimental. However, in adjustable regimens single resistance is suppressed and cycling is successful in most settings. Both a meta-regression and our theoretical model indicate that "adjustable cycling" is especially useful to suppress emergence of multiple resistance. While our model predicts that cycling periods of one month perform well, we expect that too long cycling periods are detrimental. Our results suggest that "adjustable cycling" suppresses multiple resistance and warrants further investigations that allow comparing various diseases and hospital settings.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organisation (2005) Global patient safety challenge: clean care is safer care. In: World Alliance for Patient Safety, editor. Geneva.
-
- Livermore DM (2003) Bacterial resistance: origins, epidemiology, and impact. Clin Infect Dis 36: S11–23. - PubMed
-
- Lambert ML, Suetens C, Savey A, Palomar M, Hiesmayr M, et al. (2011) Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis 11: 30–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

