The Dynamics of Morphogenesis in the Early Mouse Embryo
- PMID: 24968703
- PMCID: PMC4277506
- DOI: 10.1101/cshperspect.a015867
The Dynamics of Morphogenesis in the Early Mouse Embryo
Abstract
SUMMARYOver the past two decades, our understanding of mouse development from implantation to gastrulation has grown exponentially with an upsurge of genetic, molecular, cellular, and morphogenetic information. New discoveries have exalted the role of extraembryonic tissues in orchestrating embryonic patterning and axial specification. At the same time, the identification of unexpected morphogenetic processes occurring during mouse gastrulation has challenged established dogmas and brought new insights into the mechanisms driving germ layer formation. In this article, we summarize the key findings that have reinvigorated the contemporary view of early postimplantation mammalian development.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

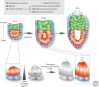
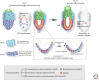
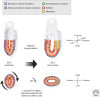

References
-
- Ang S-L, Constam DB. 2004. A gene network establishing polarity in the early mouse embryo. Dev Biol 15: 555–561. - PubMed
-
- Arnold SJ, Robertson EJ. 2009. Making a commitment: Cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol 10: 91–103. - PubMed
-
- Barrow JR, Howell WD, Rule M, Hayashi S, Thomas KR, Capecchi MR, McMahon AP. 2007. Wnt3 signaling in the epiblast is required for proper orientation of the anteroposterior axis. Dev Biol 312: 312–320. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
