Bazedoxifene versus oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis at higher risk of fracture: a network meta-analysis
- PMID: 24969003
- PMCID: PMC5583519
- DOI: 10.1016/j.jval.2014.01.008
Bazedoxifene versus oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis at higher risk of fracture: a network meta-analysis
Abstract
Objective: To compare the efficacy of bazedoxifene and oral bisphosphonates for the prevention of nonvertebral fractures (NVFs) in women with higher risk of postmenopausal osteoporosis (i.e., the Fracture Risk Assessment Tool [FRAX] score ≥ 20%), based on currently available evidence from randomized controlled trials.
Methods: Randomized controlled trials evaluating the NVF relative risk reduction (RRR) with oral bisphosphonates or bazedoxifene were identified by a systematic literature review and combined by means of a network meta-analysis. A subgroup of patients with a FRAX score of 20% or more in the bazedoxifene phase III osteoporosis study was selected as the population of interest on the basis of the bazedoxifene label. In one analysis (analysis 1), the placebo response of the subgroup with a FRAX score of 20% or more was the benchmark to select comparable bisphosphonate trials. Additional analyses incorporated the aggregate data from the bisphosphonate trials with all the FRAX subgroups (analysis 2) or with the individual patient data from the bazedoxifene trial (analysis 3).
Results: Nine identified bisphosphonate trials (alendronate, ibandronate, risedronate; N = 23,440 patients) with a similar placebo response as observed for the subgroup of high risk patients in the bazedoxifene trial were included in analysis 1. The results of the network meta-analysis of this study set suggest that bazedoxifene is expected to have an RRR of 0.43 (95% credible interval [CrI] -0.19 to 0.72) versus alendronate, 0.58 (95% CrI 0.05-0.81) versus ibandronate, and 0.39 (95% CrI -0.29 to 0.70) versus risedronate. Analyses in which treatment effects with bisphosphonates were projected to a population with a FRAX score of 20% or more with meta-regression approaches (analysis 2 and analysis 3) provide similar findings.
Conclusion: Based on an indirect comparison of randomized trials, bazedoxifene is expected to have at least a comparable RRR of NVF as alendronate, ibandronate, and risedronate in women with higher risk of postmenopausal osteoporosis.
Keywords: meta-analysis; osteoporosis; systematic review; treatment comparisons.
Copyright © 2014 International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Published by Elsevier Inc. All rights reserved.
Figures

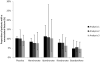
References
-
- Deal CL. Osteoporosis: prevention, diagnosis, and management. Am J Med. 1997;102(Suppl):35S–9S. - PubMed
-
- Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–75. - PubMed
-
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33. - PubMed
-
- Kanis JA, Johnell O. Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int. 2005;16:229–38. - PubMed
-
- Stevenson M, Jones ML, De Nigris E, et al. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess. 2005;9:100–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

