Shape-controllable formation of poly-imidazolium salts for stable palladium N-heterocyclic carbene polymers
- PMID: 24969738
- PMCID: PMC5381546
- DOI: 10.1038/srep05478
Shape-controllable formation of poly-imidazolium salts for stable palladium N-heterocyclic carbene polymers
Abstract
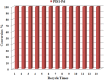
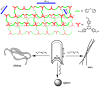
The imidazolium-based main-chain organic polymers are one of promising platforms in heterogeneous catalysis, the size and outer morphology of polymer particles are known to have important effects on their physical properties and catalytic applications, but main-chain ionic polymers usually generate amorphous or spherical particles. Herein, we presented a versatile and facile synthetic route for size- and shape-controllable synthesis of main-chain poly-imidazolium particles. The wire-shaped, spherical and ribbon-shaped morphologies of poly-imidazolium particles were readily synthesized through quaternization of bis-(imidazol-1-yl)methane and 2,4,6-tris(4-(bromomethyl)phenyl)-1,3,5-triazine, and the modification of their size and morphology were realized through adjusting solvent polarity, solubility, concentration and temperatures. The direct complexation of the particles with Pd(OAc)2 produced ionic polymers containing palladium N-heterocyclic carbene units (NHCs) with intactness of original morphologies. The particle morphologies have a significant effect on catalytic performances. Wire-shaped palladium-NHC polymer shows excellent catalytic activity and recyclabilty in heterogeneous Suzuki-Miyaura cross-coupling reaction.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Valente C. et al. The development of bulky palladium NHC complexes for the most-challenging cross-coupling reactions. Angew. Chem. Int. Ed. 51, 3314–3332 (2012). - PubMed
-
- Lin J. C. Y. et al. Coinage metal-N-Heterocyclic carbene complexes. Chem. Rev. 109, 3561–3598 (2009). - PubMed
-
- Ranganath K. V. S., Onitsuka S., Kumar A. K. & Inanaga J. Recent progress of N-heterocyclic carbenes in heterogeneous catalysis. Catal. Sci. Technol. 3, 2161–2181 (2013).
-
- Schwarz J. et al. Polymer-supported Carbene complexes of palladium: Well-defined, air-stable, recyclable catalysts for the heck reaction. Chem. Eur. J. 6, 1773–1780 (2000). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

