Resistance to thyroid hormone caused by a mutation in thyroid hormone receptor (TR)α1 and TRα2: clinical, biochemical, and genetic analyses of three related patients
- PMID: 24969835
- PMCID: PMC5989926
- DOI: 10.1016/S2213-8587(14)70111-1
Resistance to thyroid hormone caused by a mutation in thyroid hormone receptor (TR)α1 and TRα2: clinical, biochemical, and genetic analyses of three related patients
Erratum in
- Lancet Diabetes Endocrinol. 2014 Aug;2(8):e14
Abstract
Background: The thyroid hormone receptor α gene (THRA) transcript is alternatively spliced to generate either thyroid hormone receptor (TR)α1 or a non-hormone-binding variant protein, TRα2, the function of which is unknown. Here, we describe the first patients identified with a mutation in THRA that affects both TRα1 and TRα2, and compare them with patients who have resistance to thyroid hormone owing to a mutation affecting only TRα1, to delineate the relative roles of TRα1 and TRα2.
Methods: We did clinical, biochemical, and genetic analyses of an index case and her two sons. We assessed physical and radiological features, thyroid function, physiological and biochemical markers of thyroid hormone action, and THRA sequence.
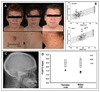
Findings: The patients presented in childhood with growth failure, developmental delay, and constipation, which improved after treatment with thyroxine, despite normal concentrations of circulating thyroid hormones. They had similar clinical (macrocephaly, broad faces, skin tags, motor dyspraxia, slow speech), biochemical (subnormal ratio of free thyroxine:free tri-iodothyronine [T3], low concentration of total reverse T3, high concentration of creatine kinase, mild anaemia), and radiological (thickened calvarium) features to patients with TRα1-mediated resistance to thyroid hormone, although our patients had a heterozygous mis-sense mutation (Ala263Val) in both TRα1 and TRα2 proteins. The Ala263Val mutant TRα1 inhibited the transcriptional function of normal receptor in a dominant-negative fashion. By contrast, function of Ala263Val mutant TRα2 matched its normal counterpart. In vitro, high concentrations of T3 restored transcriptional activity of Ala263Val mutant TRα1, and reversed the dominant-negative inhibition of its normal counterpart. High concentrations of T3 restored expression of thyroid hormone-responsive target genes in patient-derived blood cells.
Interpretation: TRα1 seems to be the principal functional product of the THRA gene. Thyroxine treatment alleviates hormone resistance in patients with mutations affecting this gene, possibly ameliorating the phenotype. These findings will help the diagnosis and treatment of other patients with resistance to thyroid hormone resulting from mutations in THRA.
Funding: Wellcome Trust, NIHR Cambridge Biomedical Research Centre, Marie Curie Actions, Foundation for Development of Internal Medicine in Europe.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


Comment in
-
Thyroid function: new mutation in human TRα proteins.Nat Rev Endocrinol. 2014 Sep;10(9):511. doi: 10.1038/nrendo.2014.109. Epub 2014 Jul 15. Nat Rev Endocrinol. 2014. PMID: 25022813 No abstract available.
References
-
- Pessemesse L, Schlernitzauer A, Sar C, et al. Depletion of the p43 mitochondrial T3 receptor in mice affects skeletal muscle development and activity. The FASEB Journal. 2012;26:748–756. - PubMed
-
- Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993;14:184–193. - PubMed
-
- Horlein AJ, Heinzel T, Rosenfeld MG. Gene regulation by thyroid hormone receptors. Curr Opin Endocrinol Diabetes. 1996;3:412–416.
-
- Refetoff S, Dunitrescu AM. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Practice and Research Clinical Endocrinology and Metabolism. 2007;21:277–305. - PubMed
-
- Gurnell M, Visser TJ, Beck-Peccoz P, Chatterjee VK. Resistance to Thyroid Hormone. In: Jameson JL, de Groot LJ, editors. Endocrinology. 6th edition. Saunders; Philadelphia: 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

