Development and Application of Multidimensional HPLC Mapping Method for O-linked Oligosaccharides
- PMID: 24970123
- PMCID: PMC4030830
- DOI: 10.3390/biom1010048
Development and Application of Multidimensional HPLC Mapping Method for O-linked Oligosaccharides
Abstract
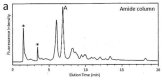
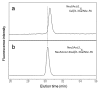
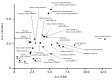
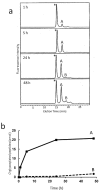
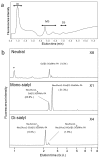
Glycosylation improves the solubility and stability of proteins, contributes to the structural integrity of protein functional sites, and mediates biomolecular recognition events involved in cell-cell communications and viral infections. The first step toward understanding the molecular mechanisms underlying these carbohydrate functionalities is a detailed characterization of glycan structures. Recently developed glycomic approaches have enabled comprehensive analyses of N-glycosylation profiles in a quantitative manner. However, there are only a few reports describing detailed O-glycosylation profiles primarily because of the lack of a widespread standard method to identify O-glycan structures. Here, we developed an HPLC mapping method for detailed identification of O-glycans including neutral, sialylated, and sulfated oligosaccharides. Furthermore, using this method, we were able to quantitatively identify isomeric products from an in vitro reaction catalyzed by N-acetylglucosamine-6O-sulfotransferases and obtain O-glycosylation profiles of serum IgA as a model glycoprotein.
Figures

References
-
- Sharon N. Lectins: Carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 2007;282:2753–2764. - PubMed
-
- Kawashima H., Petryniak B., Hiraoka N., Mitoma J., Huckaby V., Nakayama J., Uchimura K., Kadomatsu K., Muramatsu T., Lowe J.B., Fukuda M. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat. Immunol. 2005;6:1096–1104. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

