Mortalin, apoptosis, and neurodegeneration
- PMID: 24970131
- PMCID: PMC4030873
- DOI: 10.3390/biom2010143
Mortalin, apoptosis, and neurodegeneration
Abstract
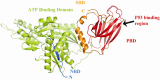
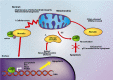
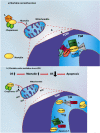
Mortalin is a highly conserved heat-shock chaperone usually found in multiple subcellular locations. It has several binding partners and has been implicated in various functions ranging from stress response, control of cell proliferation, and inhibition/prevention of apoptosis. The activity of this protein involves different structural and functional mechanisms, and minor alterations in its expression level may lead to serious biological consequences, including neurodegeneration. In this article we review the most current data associated with mortalin's binding partners and how these protein-protein interactions may be implicated in apoptosis and neurodegeneration. A complete understanding of the molecular pathways in which mortalin is involved is important for the development of therapeutic strategies for cancer and neurodegenerative diseases.
Figures


References
-
- Wadhwa R., Kaul S.C., Ikawa Y., Sugimoto Y. Identification of a novel member of mouse hsp70 family. Its association with cellular mortal phenotype. J. Biol. Chem. 1993;268:6615–6621. - PubMed
-
- Bhattacharyya T., Karnezis A.N., Murphy S.P., Hoang T., Freeman B.C., Phillips B., Morimoto R.I. Cloning and subcellular localization of human mitochondrial hsp70. J. Biol. Chem. 1995;270:1705–1710. - PubMed
-
- Webster T.J., Naylor D.J., Hartman D.J., Hoj P.B., Hoogenraad N.J. cDNA cloning and efficient mitochondrial import of pre-mtHSP70 from rat liver. DNA Cell Biol. 1994;13:1213–1220. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

