Application of Metabolic 13C Labeling in Conjunction with High-Field Nuclear Magnetic Resonance Spectroscopy for Comparative Conformational Analysis of High Mannose-Type Oligosaccharides
- PMID: 24970159
- PMCID: PMC4030882
- DOI: 10.3390/biom3010108
Application of Metabolic 13C Labeling in Conjunction with High-Field Nuclear Magnetic Resonance Spectroscopy for Comparative Conformational Analysis of High Mannose-Type Oligosaccharides
Abstract
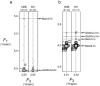
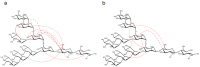
High mannose-type oligosaccharides are enzymatically trimmed in the endoplasmic reticulum, resulting in various processing intermediates with exposed glycotopes that are recognized by a series of lectins involved in glycoprotein fate determination in cells. Although recent crystallographic data have provided the structural basis for the carbohydrate recognition of intracellular lectins, atomic information of dynamic oligosaccharide conformations is essential for a quantitative understanding of the energetics of carbohydrate-lectin interactions. Carbohydrate NMR spectroscopy is useful for characterizing such conformational dynamics, but often hampered by poor spectral resolution and lack of recombinant techniques required to produce homogeneous glycoforms. To overcome these difficulties, we have recently developed a methodology for the preparation of a homogeneous high mannose-type oligosaccharide with 13C labeling using a genetically engineered yeast strain. We herein successfully extended this method to result in the overexpression of 13C-labeled Man9GlcNAc2 (M9) with a newly engineered yeast strain with the deletion of four genes involved in N-glycan processing. This enabled high-field NMR analyses of 13C-labeled M9 in comparison with its processing product lacking the terminal mannose residue ManD2. Long-range NOE data indicated that the outer branches interact with the core in both glycoforms, and such foldback conformations are enhanced upon the removal of ManD2. The observed conformational variabilities might be significantly associated with lectins and glycan-trimming enzymes.
Figures



References
-
- von der Lieth C.W., Siebert H.C., Kožár T., Burchert M., Frank M., Gilleron M., Kaltner H., Kayser G., Tajkhorshid E., Bovin N.V., Vliegenthart J.F.G., Gabius H.J. Lectin ligands: new insights into their conformations and their dynamic behavior and the discovery of conformer selection by lectins. Acta anatomica. 1998;161:91–109. doi: 10.1159/000046452. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

