Regulation of cytoskeleton organization by sphingosine in a mouse cell model of progressive ovarian cancer
- PMID: 24970173
- PMCID: PMC4030958
- DOI: 10.3390/biom3030386
Regulation of cytoskeleton organization by sphingosine in a mouse cell model of progressive ovarian cancer
Abstract
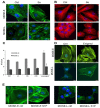
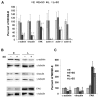
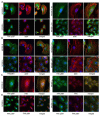
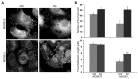
Ovarian cancer is a multigenic disease and molecular events driving ovarian cancer progression are not well established. We have previously reported the dysregulation of the cytoskeleton during ovarian cancer progression in a syngeneic mouse cell model for progressive ovarian cancer. In the present studies, we investigated if the cytoskeleton organization is a potential target for chemopreventive treatment with the bioactive sphingolipid metabolite sphingosine. Long-term treatment with non-toxic concentrations of sphingosine but not other sphingolipid metabolites led to a partial reversal of a cytoskeleton architecture commonly associated with aggressive cancer phenotypes towards an organization reminiscent of non-malignant cell phenotypes. This was evident by increased F-actin polymerization and organization, a reduced focal adhesion kinase expression, increased a-actinin and vinculin levels which together led to the assembly of more mature focal adhesions. Downstream focal adhesion signaling, the suppression of myosin light chain kinase expression and hypophosphorylation of its targets were observed after treatment with sphingosine. These results suggest that sphingosine modulate the assembly of actin stress fibers via regulation of focal adhesions and myosin light chain kinase. The impact of these events on suppression of ovarian cancer by exogenous sphingosine and their potential as molecular markers for treatment efficacy warrants further investigation.
Figures

Similar articles
-
Changes in gene expression and cellular architecture in an ovarian cancer progression model.PLoS One. 2011 Mar 3;6(3):e17676. doi: 10.1371/journal.pone.0017676. PLoS One. 2011. PMID: 21390237 Free PMC article.
-
Roles of bioactive sphingolipid metabolites in ovarian cancer cell biomechanics.Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:2436-9. doi: 10.1109/EMBC.2012.6346456. Annu Int Conf IEEE Eng Med Biol Soc. 2012. PMID: 23366417
-
Bioactive sphingolipid metabolites modulate ovarian cancer cell structural mechanics.Integr Biol (Camb). 2013 Nov;5(11):1385-92. doi: 10.1039/c3ib40121a. Epub 2013 Sep 20. Integr Biol (Camb). 2013. PMID: 24056950 Free PMC article.
-
Disruption of actin-myosin interactions results in the inhibition of focal adhesion assembly in Xenopus XR1 glial cells.Glia. 1999 May;26(3):245-59. Glia. 1999. PMID: 10340765
-
Integrin-mediated cell adhesion: the cytoskeletal connection.Biochem Soc Symp. 1999;65:79-99. Biochem Soc Symp. 1999. PMID: 10320934 Review.
Cited by
-
Ovarian tumor-initiating cells display a flexible metabolism.Exp Cell Res. 2014 Oct 15;328(1):44-57. doi: 10.1016/j.yexcr.2014.08.028. Epub 2014 Aug 27. Exp Cell Res. 2014. PMID: 25172556 Free PMC article.
-
Quantitative biophysical metrics for rapid evaluation of ovarian cancer metastatic potential.Mol Biol Cell. 2022 May 15;33(6):ar55. doi: 10.1091/mbc.E21-08-0419. Epub 2022 Jan 5. Mol Biol Cell. 2022. PMID: 34985924 Free PMC article.
-
Progression-Mediated Changes in Mitochondrial Morphology Promotes Adaptation to Hypoxic Peritoneal Conditions in Serous Ovarian Cancer.Front Oncol. 2021 Jan 13;10:600113. doi: 10.3389/fonc.2020.600113. eCollection 2020. Front Oncol. 2021. PMID: 33520711 Free PMC article.
-
Fluid shear stress impacts ovarian cancer cell viability, subcellular organization, and promotes genomic instability.PLoS One. 2018 Mar 22;13(3):e0194170. doi: 10.1371/journal.pone.0194170. eCollection 2018. PLoS One. 2018. PMID: 29566010 Free PMC article.
-
Biomechanical profile of cancer stem-like/tumor-initiating cells derived from a progressive ovarian cancer model.Nanomedicine. 2014 Jul;10(5):1013-9. doi: 10.1016/j.nano.2013.12.009. Epub 2014 Jan 6. Nanomedicine. 2014. PMID: 24407147 Free PMC article.
References
-
- Howlader N., Noone A.M., Krapho M., Neyman N., Aminou R., Waldron W., Altekruse S.F., Kosary C.L., Ruhl J., Tatalovich Z., et al. National Cancer Institute; Bethesda, MD, USA: 2011. [(accessed on July 2, 2013)]. SEER Cancer Statistics Review, 1975–2008. Available online: http://seer.cancer.gov/csr/1975_2008/
-
- Barkan D., Kleinman H., Simmons J.L., Asmussen H., Kamaraju A.K., Hoenorhoff M.J., Liu Z.Y., Costes S.V., Cho E.H., Lockett S., et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. - DOI - PMC - PubMed
-
- Pawlak G., Helfman D.M. Cytoskeletal changes in cell transformation and tumorigenesis. Curr. Opin. Genet. Dev. 2001;11:41–47. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

