Biophysical Characterization of α-Synuclein and Rotenone Interaction
- PMID: 24970188
- PMCID: PMC4030960
- DOI: 10.3390/biom3030703
Biophysical Characterization of α-Synuclein and Rotenone Interaction
Abstract
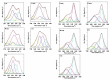
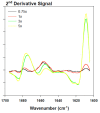
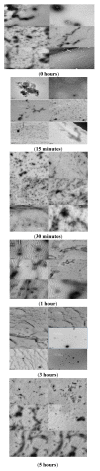
Previous studies revealed that pesticides interact with α-synuclein and accelerate the rate of fibrillation. These results are consistent with the prevailing hypothesis that the direct interaction of α-synuclein with pesticides is one of many suspected factors leading to α-synuclein fibrillation and ultimately to Parkinson's disease. In this study, the biophysical properties and fibrillation kinetics of α-synuclein in the presence of rotenone were investigated and, more specifically, the effects of rotenone on the early-stage misfolded forms of α-synuclein were considered. The thioflavine T (ThT) fluorescence assay studies provide evidence that early-phase misfolded α-synuclein forms are affected by rotenone and that the fibrillation process is accelerated. Further characterization by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) shows that rotenone increases the amount of ordered secondary structure in this intrinsically disordered protein. Morphological characterization by transmission electron microscopy (TEM) and atomic force microscopy (AFM) provide visualization of the differences in the aggregated α-synuclein species developing during the early kinetics of the fibrillation process in the absence and presence of rotenone. We believe that these data provide useful information for a better understanding of the molecular basis of rotenone-induced misfolding and aggregation of α-synuclein.
Figures






References
-
- Uversky V.N. Neuropathology and neurochemistry of Parkinson’s disease: The never-ending story or the story with no beginning? Minerva Psichiatr. 2009;50:1–26.
-
- Uversky V.N., Fink A.L. Biophysical Properties of Human Alpha-Synuclein and its Role in Parkinson’s Disease. In: Pandalai S.G., editor. Recent Research Developments in Proteins. Transworld Research Network; Kerala, India: 2002. pp. 153–186.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

