Biocatalysis for biobased chemicals
- PMID: 24970192
- PMCID: PMC4030974
- DOI: 10.3390/biom3040812
Biocatalysis for biobased chemicals
Abstract
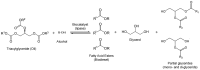
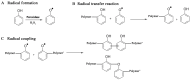
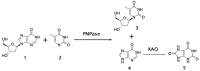
The design and development of greener processes that are safe and friendly is an irreversible trend that is driven by sustainable and economic issues. The use of Biocatalysis as part of a manufacturing process fits well in this trend as enzymes are themselves biodegradable, require mild conditions to work and are highly specific and well suited to carry out complex reactions in a simple way. The growth of computational capabilities in the last decades has allowed Biocatalysis to develop sophisticated tools to understand better enzymatic phenomena and to have the power to control not only process conditions but also the enzyme's own nature. Nowadays, Biocatalysis is behind some important products in the pharmaceutical, cosmetic, food and bulk chemicals industry. In this review we want to present some of the most representative examples of industrial chemicals produced in vitro through enzymatic catalysis.
Figures
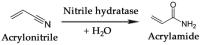

References
-
- Council of European Union . En Route to the Knowledge-Based Bio-Economy. German Presidency of the Council of European Union; Cologne, Germany: 2007.
-
- Sandoval G., Plou F.G. Obtención enzimática de compuestos bioactivos a partir de recursos naturales iberoamericanos. Consejo Superior de Instigaciones Cientificas (CSIC); Madrid, Spain: 2012. pp. 1–336.
-
- Uchiyama T., Niwa S., Tanaka K. Purification and properties of Arthrobacter ureafaciens inulase II. Biochim. Biophys. Acta. 1973;315:412–420. doi: 10.1016/0005-2744(73)90271-4. - DOI
-
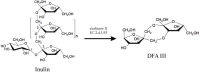
- Kim G.E., Lee S. Abstracts of the World Congress on Biotechnology. Volume 272 DECHEMA; Berlin, Germany: 2000. Efficient production of DFA III (di-d-fructofuranose-dianhydride) from chicory root.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

