Control of cell differentiation by mitochondria, typically evidenced in dictyostelium development
- PMID: 24970198
- PMCID: PMC4030964
- DOI: 10.3390/biom3040943
Control of cell differentiation by mitochondria, typically evidenced in dictyostelium development
Abstract
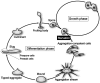
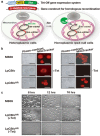
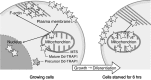
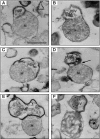
In eukaryotic cells, mitochondria are self-reproducing organelles with their own DNA and they play a central role in adenosine triphosphate (ATP) synthesis by respiration. Increasing evidence indicates that mitochondria also have critical and multiple functions in the initiation of cell differentiation, cell-type determination, cell movement, and pattern formation. This has been most strikingly realized in development of the cellular slime mold Dictyostelium. For example, the expression of the mitochondrial ribosomal protein S4 (mt-rps4) gene is required for the initial differentiation. The Dictyostelium homologue (Dd-TRAP1) of TRAP-1 (tumor necrosis receptor-associated protein 1), a mitochondrial molecular chaperone belonging to the Hsp90 family, allows the prompt transition of cells from growth to differentiation through a novel prestarvation factor (PSF-3) in growth medium. Moreover, a cell-type-specific organelle named a prespore-specific vacuole (PSV) is constructed by mitochondrial transformation with the help of the Golgi complex. Mitochondria are also closely involved in a variety of cellular activities including CN-resistant respiration and apoptosis. These mitochondrial functions are reviewed in this article, with special emphasis on the regulation of Dictyostelium development.
Figures


References
-
- Hartwell L.H., Weinert T.A. Checkpoints: Controls that ensure the order of cell cycle events. Science. 1989;246:629–634. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

