The oral iron chelator deferiprone protects against systemic iron overload-induced retinal degeneration in hepcidin knockout mice
- PMID: 24970260
- PMCID: PMC4106252
- DOI: 10.1167/iovs.14-14568
The oral iron chelator deferiprone protects against systemic iron overload-induced retinal degeneration in hepcidin knockout mice
Abstract
Purpose: To investigate the retinal-protective effects of the oral iron chelator deferiprone (DFP) in mice lacking the iron regulatory hormone hepcidin (Hepc). These Hepc knockout (KO) mice have age-dependent systemic and retinal iron accumulation leading to retinal degeneration.
Methods: Hepc KO mice were given DFP in drinking water from age 6 to 18 months. They were then compared to Hepc KO mice not receiving DFP by fundus imaging, electroretinography (ERG), histology, immunofluorescence, and quantitative PCR to investigate the protective effect of DFP against retinal and retinal pigment epithelial (RPE) degeneration.
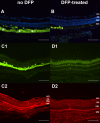
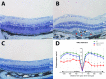
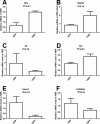
Results: In Hepc KO mice, DFP diminished RPE depigmentation and autofluorescence on fundus imaging. Autofluorescence in the RPE layer in cryosections was significantly diminished by DFP, consistent with the fundus images. Immunolabeling with L-ferritin and transferrin receptor antibodies showed a decreased signal for L-ferritin in the inner retina and RPE cells and an increased signal for transferrin receptor in the inner retina, indicating diminished retinal iron levels with DFP treatment. Plastic sections showed that photoreceptor and RPE cells were well preserved in Hepc KO mice treated with DFP. Consistent with photoreceptor protection, the mRNA level of rhodopsin was significantly higher in retinas treated with DFP. The mRNA levels of oxidative stress-related genes heme oxygenase-1 and catalase were significantly lower in DFP-treated Hepc KO retinas. Finally, ERG rod a- and b- and cone b-wave amplitudes were significantly higher in DFP-treated mice.
Conclusions: Long-term treatment with the oral iron chelator DFP diminished retinal and RPE iron levels and oxidative stress, providing significant protection against retinal degeneration caused by chronic systemic iron overload in Hepc KO mice. This indicates that iron chelation could be a long-term preventive treatment for retinal disease involving iron overload and oxidative stress.
Keywords: deferiprone; hepcidin; oxidative stress; retinal degeneration.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures


Similar articles
-
The oral iron chelator deferiprone protects against iron overload-induced retinal degeneration.Invest Ophthalmol Vis Sci. 2011 Feb 16;52(2):959-68. doi: 10.1167/iovs.10-6207. Invest Ophthalmol Vis Sci. 2011. PMID: 21051716 Free PMC article.
-
Systemic administration of the iron chelator deferiprone protects against light-induced photoreceptor degeneration in the mouse retina.Free Radic Biol Med. 2012 Jul 1;53(1):64-71. doi: 10.1016/j.freeradbiomed.2012.04.020. Epub 2012 May 1. Free Radic Biol Med. 2012. PMID: 22579919 Free PMC article.
-
Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice.Invest Ophthalmol Vis Sci. 2011 Jan 5;52(1):109-18. doi: 10.1167/iovs.10-6113. Invest Ophthalmol Vis Sci. 2011. PMID: 20811044 Free PMC article.
-
Monitoring long-term efficacy of iron chelation treatment with biomagnetic liver susceptometry.Ann N Y Acad Sci. 2005;1054:350-7. doi: 10.1196/annals.1345.043. Ann N Y Acad Sci. 2005. PMID: 16339683 Review.
-
Iron homeostasis and toxicity in retinal degeneration.Prog Retin Eye Res. 2007 Nov;26(6):649-73. doi: 10.1016/j.preteyeres.2007.07.004. Epub 2007 Aug 11. Prog Retin Eye Res. 2007. PMID: 17921041 Free PMC article. Review.
Cited by
-
Dysregulation of Mitochondrial Iron Regulators as a Basis of Iron-Mediated Retinal Degeneration in Rats.Neurotox Res. 2025 Jun 13;43(3):29. doi: 10.1007/s12640-025-00752-4. Neurotox Res. 2025. PMID: 40512285 No abstract available.
-
Local synthesis of hepcidin in the anterior segment of the eye: A novel observation with physiological and pathological implications.Exp Eye Res. 2020 Jan;190:107890. doi: 10.1016/j.exer.2019.107890. Epub 2019 Dec 4. Exp Eye Res. 2020. PMID: 31811823 Free PMC article.
-
Intraocular iron injection induces oxidative stress followed by elements of geographic atrophy and sympathetic ophthalmia.Aging Cell. 2021 Nov;20(11):e13490. doi: 10.1111/acel.13490. Epub 2021 Oct 9. Aging Cell. 2021. PMID: 34626070 Free PMC article.
-
Molecular mechanisms of NMDA excitotoxicity in the retina.Sci Rep. 2023 Oct 27;13(1):18471. doi: 10.1038/s41598-023-45855-0. Sci Rep. 2023. PMID: 37891222 Free PMC article.
-
Berberine protects against light-induced photoreceptor degeneration in the mouse retina.Exp Eye Res. 2016 Apr;145:1-9. doi: 10.1016/j.exer.2015.10.005. Epub 2015 Oct 22. Exp Eye Res. 2016. PMID: 26475979 Free PMC article.
References
-
- Hahn P, Milam AH, Dunaief JL. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch's membrane. Arch Ophthalmol. 2003; 121: 1099–1105 - PubMed
-
- Vulpe CD, Kuo Y-M, Murphy TL, et al. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999; 21: 195–199 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

