Sulfatase 1 (hSulf-1) reverses basic fibroblast growth factor-stimulated signaling and inhibits growth of hepatocellular carcinoma in animal model
- PMID: 24970807
- PMCID: PMC4148119
- DOI: 10.18632/oncotarget.2078
Sulfatase 1 (hSulf-1) reverses basic fibroblast growth factor-stimulated signaling and inhibits growth of hepatocellular carcinoma in animal model
Abstract
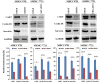
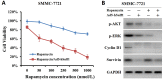
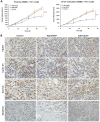
The human sulfatase 1 (hSulf-1) gene encodes an endosulfatase that functions to inhibit the heparin-binding growth factor signaling, including the basic fibroblast growth factor (bFGF)-mediated pathway, by desulfating the cell surface heparan sulfate proteoglycans (HSPGs). bFGF could stimulate cell cycle progression and inhibit cell apoptosis, this biological effect can be reversed by hSulf-1. However, molecular mechanisms have not been fully reported. In the current study, by reactivation of hSulf-1 expression and function in the hSulf-1-negative hepatocellular carcinoma (HCC) cell lines and HCC xenograft tumors, we found that hSulf-1 blocked the bFGF effect on the promotion of cell cycle and inhibition of apoptosis. The bFGF-stimulated activation of protein kinase B (AKT) and extracellular signal-regulated kinase (ERK) pathways was suppressed by hSulf-1, which led to a decreased expression of the target genes Cyclin D1 and Survivin, then finally induced cell cycle arrest and apoptosis in HCC cells. Our data suggested that hSulf-1 may be a suitable target for cancer therapy.
Figures



References
-
- Wang TR, Yan LY, Yan J, Lu CL, Xia X, Yin TL, Zhu XH, Gao JM, Ding T, Hu WH, Guo HY, Li R, Qiao J. Basic fibroblast growth factor promotes the development of human ovarian early follicles during growth in vitro. >Hum Reprod. 2014. doi: 10.1093/humrep/det465. - PubMed
-
- Park DJ, Yoon C, Thomas N, Ku GY, Janjigian YY, Kelsen DP, Ilson DH, Goodman KA, Tang LH, Strong VE, Coit DG, Yoon SS. Prognostic Significance of Targetable Angiogenic and Growth Factors in Patients Undergoing Resection for Gastric and Gastroesophageal Junction Cancers. Ann Surg Oncol. 2013. doi: 10.1245/s10434-013-3429-0. - PubMed
-
- Chiorean EG, Sweeney C, Youssoufian H, Qin A, Dontabhaktuni A, Loizos N, Nippgen J, Amato R. A phase I study of olaratumab, an anti-platelet-derived growth factor receptor alpha (PDGFRα) monoclonal antibody, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014. doi: 10.1007/s00280-014-2389-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

