The subcellular distribution and function of MTA1 in cancer differentiation
- PMID: 24970816
- PMCID: PMC4148129
- DOI: 10.18632/oncotarget.2095
The subcellular distribution and function of MTA1 in cancer differentiation
Abstract
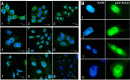
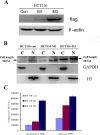
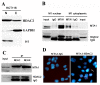
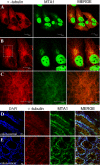
The functions and mechanisms of metastasis-associated protein 1 (MTA1) in cancer progression are still unclear due to a lagged recognition of the subcellular localization. In the present study, using multiple molecular technologies we confirmed for the first time that MTA1 localizes to the nucleus, cytoplasm and nuclear envelope. MTA1 is primarily localized in the nucleus of normal adult tissues but in the cytoplasm of embryonic tissues. While in colon cancer, both distributions have been described. Further investigation revealed that MTA1 localizes on the nuclear envelope in a translocated promoter region (TPR)-dependent manner, while in the cytoplasm, MTA1 shows an obvious localization on microtubules. Both nuclear and cytoplasmic MTA1 are associated with cancer progression. However, these functions may be associated with different mechanisms because only nuclear MTA1 has been associated with cancer differentiation. Overexpression of MTA1 in HCT116 cells inhibited differentiation and promoted proliferation, whereas MTA1 knockdown resulted in cell differentiation and death. Theses results not only suggest that nuclear MTA1 is a good marker for cancer differentiation diagnosis and a potential target for the treatment of cancers but also reveal the necessity to differentially examine the functions of nuclear and cytoplasmic MTA1.
Figures



References
-
- Toh Y, Pencil SD, Nicolson GL. A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J Biol Chem. 1994;269:22958–22963. - PubMed
-
- Toh Y, Pencil SD, Nicolson GL. Analysis of the complete sequence of the novel metastasis-associated candidate gene, mta1, differentially expressed in mammary adenocarcinoma and breast cancer cell lines. Gene. 1995;159:97–104. - PubMed
-
- Pencil SD, Toh Y, Nicolson GL. Candidate metastasis-associated genes of the rat 13762NF mammary adenocarcinoma. Breast Cancer Res Treat. 1993;25:165–174. - PubMed
-
- Toh Y, Nicolson GL. The role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implications. Clin Exp Metastasis. 2009;26:215–227. - PubMed
-
- Xue Y, Wong J, Moreno GT, Young MK, Cote J, Wang W. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol Cell. 1998;2:851–861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

