Listeria monocytogenes induces IFNβ expression through an IFI16-, cGAS- and STING-dependent pathway
- PMID: 24970844
- PMCID: PMC4194099
- DOI: 10.15252/embj.201488029
Listeria monocytogenes induces IFNβ expression through an IFI16-, cGAS- and STING-dependent pathway
Abstract
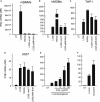
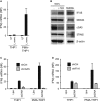
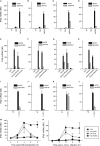
Listeria monocytogenes is a gram-positive facultative intracellular bacterium, which replicates in the cytoplasm of myeloid cells. Interferon β (IFNβ) has been reported to play an important role in the mechanisms underlying Listeria disease. Although studies in murine cells have proposed the bacteria-derived cyclic-di-AMP to be the key bacterial immunostimulatory molecule, the mechanism for IFNβ expression during L. monocytogenes infection in human myeloid cells remains unknown. Here we report that in human macrophages, Listeria DNA rather than cyclic-di-AMP is stimulating the IFN response via a pathway dependent on the DNA sensors IFI16 and cGAS as well as the signalling adaptor molecule STING. Thus, Listeria DNA is a major trigger of IFNβ expression in human myeloid cells and is sensed to activate a pathway dependent on IFI16, cGAS and STING.
Keywords: Listeria monocytogenes; innate immunity; interferon beta.
© 2014 The Authors.
Figures



References
-
- Abdullah Z, Schlee M, Roth S, Mraheil MA, Barchet W, Bottcher J, Hain T, Geiger S, Hayakawa Y, Fritz JH, Civril F, Hopfner KP, Kurts C, Ruland J, Hartmann G, Chakraborty T, Knolle PA. RIG-I detects infection with live Listeria by sensing secreted bacterial nucleic acids. EMBO J. 2012;31:4153–4164. - PMC - PubMed
-
- Barbuddhe SB, Chakraborty T. Listeria as an enteroinvasive gastrointestinal pathogen. Curr Top Microbiol Immunol. 2009;337:173–195. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

