Surface tension in situ in flooded alveolus unaltered by albumin
- PMID: 24970853
- PMCID: PMC5243207
- DOI: 10.1152/japplphysiol.00084.2014
Surface tension in situ in flooded alveolus unaltered by albumin
Abstract
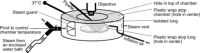
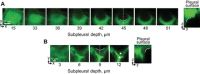
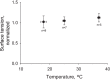
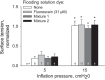
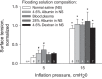
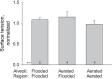
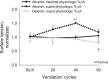
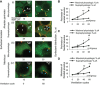
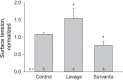
In the acute respiratory distress syndrome, plasma proteins in alveolar edema liquid are thought to inactivate lung surfactant and raise surface tension, T. However, plasma protein-surfactant interaction has been assessed only in vitro, during unphysiologically large surface area compression (%ΔA). Here, we investigate whether plasma proteins raise T in situ in the isolated rat lung under physiologic conditions. We flood alveoli with liquid that omits/includes plasma proteins. We ventilate the lung between transpulmonary pressures of 5 and 15 cmH2O to apply a near-maximal physiologic %ΔA, comparable to that of severe mechanical ventilation, or between 1 and 30 cmH2O, to apply a supraphysiologic %ΔA. We pause ventilation for 20 min and determine T at the meniscus that is present at the flooded alveolar mouth. We determine alveolar air pressure at the trachea, alveolar liquid phase pressure by servo-nulling pressure measurement, and meniscus radius by confocal microscopy, and we calculate T according to the Laplace relation. Over 60 ventilation cycles, application of maximal physiologic %ΔA to alveoli flooded with 4.6% albumin solution does not alter T; supraphysiologic %ΔA raise T, transiently, by 51 ± 4%. In separate experiments, we find that addition of exogenous surfactant to the alveolar liquid can, with two cycles of maximal physiologic %ΔA, reduce T by 29 ± 11% despite the presence of albumin. We interpret that supraphysiologic %ΔA likely collapses the interfacial surfactant monolayer, allowing albumin to raise T. With maximal physiologic %ΔA, the monolayer likely remains intact such that albumin, blocked from the interface, cannot interfere with native or exogenous surfactant activity.
Keywords: albumin; alveolar edema; plasma proteins; surface tension; surfactant.
Copyright © 2014 the American Physiological Society.
Figures

Comment in
-
Invited editorial on "Surface tension in situ in flooded alveolus unaltered by albumin".J Appl Physiol (1985). 2014 Sep 1;117(5):423-4. doi: 10.1152/japplphysiol.00631.2014. Epub 2014 Jul 18. J Appl Physiol (1985). 2014. PMID: 25038107 No abstract available.
References
-
- Ashino Y, Ying X, Dobbs LG, Bhattacharya J. [Ca2+]i oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am J Physiol Lung Cell Mol Physiol 279: L5–L13, 2000. - PubMed
-
- Bachofen H, Schürch S, Michel RP, Weibel ER. Experimental hydrostatic pulmonary edema in rabbit lungs. Morphology. Am Rev Respir Dis 147: 989–996, 1993. - PubMed
-
- Bachofen H, Schürch S, Urbinelli M, Weibel ER. Relations among alveolar surface tension, surface area, volume, and recoil pressure. J Appl Physiol 62: 1878–1887, 1987. - PubMed
-
- Bhattacharya J, Gropper MA, Staub NC. Interstitial fluid pressure gradient measured by micropuncture in excised dog lung. J Appl Physiol 56: 271–277, 1984. - PubMed
-
- Brower RG, Fessler HE. Another “negative” trial of surfactant. Time to bury this idea? Am J Respir Crit Care Med 183: 966–968, 2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

