Design of a new therapy to treat snake envenomation
- PMID: 24971000
- PMCID: PMC4069039
- DOI: 10.2147/DDDT.S65395
Design of a new therapy to treat snake envenomation
Abstract
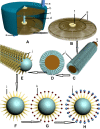
The prospective removal of snake venoms from the blood of snake-bitten patients is discussed here. Opportune neutralization of killer antigens from the blood of poisoned victims is a vital treatment step. Delays may lead to death, or cripple the patient permanently. The present procedure describes the elimination of venom antigens of a wide range of snakes from the blood of such patients. Compared to conventional treatments, the treatment is administrable in the lack of proper antivenoms, expected to be more effective with less side effects, covers a vast range of snake venoms, minimizes contact of venoms with internal tissues and organs, is applicable in patients sensitive to serum injections, has a high chance of effectiveness because there is no need to identity the snake involved to administer its specific antibody, and is capable of universal application. The principal component to this approach is a "polyvalent venom antibody column" (PVAC), which selectively traps venom antigens from blood in an extracorporeal circuit while detoxified blood returns back to the patient's body. The PVAC is intended for removal of numerous snake venom antigens in a relatively simple procedure. Detoxification is performed under the supervision of trained personnel using simple blood-circulating machines in which blood circulates from patient to PVAC and back to the patient aseptically. The device acts as a biological filter that selectively immobilizes harmful venom antigens from poisoned blood. For effective neutralization, the PVAC provides a large contact surface area with blood. The PVAC's reactive sites would consist of carbon nanotubes, on which a vast spectra of venoms' antibodies are bonded to. In this extracorporeal detoxification process, nocent antigens conjugate with their antibodies and become immobilized, and are eliminated from the poisoned patient blood. Detoxification resuscitation is expected to take 2-3 hours, when the titers of venom antigens in the blood reach harmless levels, as confirmed by sampling of the blood and appropriate serological evaluations. If conventional antivenoms do not cover the entire spectrum of venom antigens in blood, rehabilitation would be a matter of a longer period; whilst the PVAC covers the widest range of antibodies to remove the broadest range of venom antigens, the rehabilitation period would be shorter since venom antigens have been removed from the body in a few hours duration. PVACs are to be biotechnologically engineered against a wide spectra of antigens present in the venoms of the dominant poisonous snakes for a defined geographical zone; ie, a country, part of a continent, or an entire continent. As a polyvalent column, the PVAC bears a sufficient amount of venom antibodies of all snakes that pose a threat in the region. PVAC treatment would have high applicability in cases where the patient is unconscious and/or the snake identity is not clear for administration of related antivenom medication. For opportune administration, research on the use of PVACs in emergency ambulances should receive special attention. Starting in situ detoxification, such ambulances would provide more efficient resuscitations to envenomed patients.
Keywords: blood; detoxification; intoxication; polyvalent antibody; toxin; venom.
Figures
References
-
- Baride RM, Jain SD, Gaitonde BB. Biochemical studies on the toxoids of venoms of poisonous Indian snakes. Indian J Med Res. 1980;72:571–576. - PubMed
-
- Tu AT. Overview of Snake Venom Chemistry. Adv Exp Med Biol. 1996;391:37–62. - PubMed
-
- Hayes WK. Research on Biological Roles and Variation of Snake Venoms [webpage on the Internet] Loma Linda: Loma Linda University; 2014. [Accessed March 14, 2014]. Available from: http://www.llu.edu/public-health/ebs/hayes/research-c-venom.page.
-
- Chippaux JP, Williams V, White J. Snake venom variability: methods of study, results and interpretation. Toxicon. 1991;29(11):1279–1303. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

