Nanosizing of a poorly soluble drug: technique optimization, factorial analysis, and pharmacokinetic study in healthy human volunteers
- PMID: 24971006
- PMCID: PMC4069131
- DOI: 10.2147/IJN.S63395
Nanosizing of a poorly soluble drug: technique optimization, factorial analysis, and pharmacokinetic study in healthy human volunteers
Abstract
Context: Diacerein (DCN) has low aqueous solubility (3.197 mg/L) and, consequently, low oral bioavailability (35%-56%). To increase both the solubility and dissolution rate of DCN while maintaining its crystalline nature, high pressure homogenization was used but with only a few homogenization cycles preceded by a simple bottom-up technique.
Methods: The nanosuspensions of DCN were prepared using a combined bottom-up/top-down technique. Different surfactants - polyvinyl alcohol, sodium deoxycholate, and sodium dodecyl sulfate - with different concentrations were used for the stabilization of the nanosuspensions. Full factorial experimental design was employed to investigate the influence of formulation variables on nanosuspension characteristics using Design-Expert(®) Software. Particle size (PS), zeta potential, saturation solubility, in vitro dissolution, and drug crystallinity were studied. Moreover, the in vivo performance of the optimized formula was assessed by bioavailability determination in healthy human volunteers.
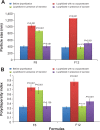
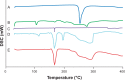
Results: The concentration of surfactant had a significant effect on both the PS and polydispersity index values. The 1% surfactant concentration showed the lowest PS and polydispersity index values compared with other concentrations. Both type and concentration of surfactant had significant effects on the zeta potential. Formula F8 (containing 1% sodium deoxycholate) and Formula F12 (containing 1% sodium dodecyl sulfate) had the highest desirability values (0.952 and 0.927, respectively). Hence, they were selected for further characterization. The saturated solubility and mean dissolution time, in the case of F8 and F12, were significantly higher than the coarse drug powder. Techniques utilized in the nanocrystals' preparation had no effect on DCN crystalline state. The selected formula (F12) showed a higher bioavailability compared to the reference market product with relative bioavailability of 131.4%.
Conclusion: The saturation solubility, in vitro dissolution rate and relative bioavailability of DCN were significantly increased after nanocrystallization. Less time and power consumption were applied by the combination of bottom-up and top-down techniques.
Keywords: diacerein; factorial analysis; high pressure homogenization; nanocrystals; pharmacokinetic study.
Figures





References
-
- Abdelbary AA, Li X, El-Nabarawi M, Elassasy A, Jasti B. Comparison of nanomilling and coprecipitation on the enhancement of in vitro dissolution rate of poorly water-soluble model drug aripiprazole. Pharm Dev Technol. 2014;19(4):491–500. - PubMed
-
- Lipinski CA. Poor aqueous solubility – an industry wide problem in drug discovery. American Pharmaceutical Review. 2002;5:82–85.
-
- Kesisoglou F, Panmai S, Wu Y. Nanosizing – oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–644. - PubMed
-
- Chen Y, Zhang GG, Neilly J, Marsh K, Mawhinney D, Sanzgiri YD. Enhancing the bioavailability of ABT-963 using solid dispersion containing Pluronic F-68. Int J Pharm. 2004;286(1–2):69–80. - PubMed
-
- Ali SM, Upadhyay SK, Maheshwari A. NMR spectroscopic study of the inclusion complex of desloratadine with β-cyclodextrin in solution. J Incl Phenom Macrocycl Chem. 2007;59(3–4):351–355.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

