Health policy model: long-term predictive results associated with the management of hepatitis C virus-induced diseases in Italy
- PMID: 24971024
- PMCID: PMC4069043
- DOI: 10.2147/CEOR.S62092
Health policy model: long-term predictive results associated with the management of hepatitis C virus-induced diseases in Italy
Abstract
Background: At present, there are no specific nationwide epidemiological studies representing the whole Italian population. This study is aimed at describing the epidemiological and economic burden that HCV will generate in the next few years in Italy. Furthermore, the impact that future anti-HCV treatments may have on the burden of disease was considered. This analysis was developed for the period 2012-2030 from the perspective of the Italian National Health Service (NHS).
Methods: A published system dynamic model was adapted for Italy in order to quantify the HCV-infected population in terms of disease progression and the associated costs from 1950 to 2030. The model structure was based on transition probabilities reflecting the natural history of the disease. In order to estimate the efficacy of current anti-HCV treatment strategies for genotypes 1 and 4, the sustained virological response (SVR) rate in registration clinical trials for both boceprevir and telaprevir was estimated. It was assumed that the efficacy for patients treated with peginterferon + ribavirin was equal to the placebo arm of a randomized clinical trial (RCT) relating to boceprevir and telaprevir. For genotypes 2/3 patients it was assumed that treatment efficacy with dual therapy was equal to a SVR rate from the literature. According to the aim of this study, only direct health care costs (hospital admissions, drugs, treatment, and care of patients) incurred by the Italian NHS have been included in the model. Costs have been extrapolated using the published scientific literature available in Italy and actualized with the 2012 ISTAT (Istituto Nazionale di Statistica) Price Index system for monetary revaluation. Three different scenarios were assumed in order to evaluate the impact of future anti-HCV treatments on the burden of disease.
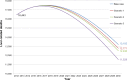
Results: Overall, in Italy, 1.2 million infected subjects were estimated in 2012. Of these, about 211,000 patients were diagnosed, while only about 11,800 subjects were actually being treated with anti-HCV drugs. A reduction of health care costs is associated with a prevalence decrease. Indeed, once the spending peak is reached during this decade (about €527 million), the model predicts a cost reduction in the following 18 years. In 2030, based on the more effective treatments currently available, the direct health care cost associated with the management of HCV patients may reach €346 million (-34.3% compared to 2012). The first scenario (new treatment in 2015 with SVR =90% and same number of treated patients) was associated with a significant reduction in HCV-induced clinical consequences (prevalence =-3%) and a decrease in direct health care expenses, corresponding to €11.1 million. The second scenario (increase in treated patients to 12,790) produced an incremental cost reduction of €7.3 million, reaching a net decrease equal to €18.4 million. In the third scenario (treated patients =16,770), a higher net direct health care cost decrease versus the base-case (€44.0 million) was estimated.
Conclusion: Our model showed that the introduction of new treatments that are more effective could result in a quasi-eradication of HCV, with a very strong reduction in prevalence.
Keywords: chronic hepatitis; cost of illness; forecast; new HCV treatment.
Figures



References
-
- World Health Organization . Sixty-third World Health Assembly. Viral hepatitis: WHA 63.18; Geneva, Switzerland: May 21, 2010.
-
- Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. - PubMed
-
- European Centre for Disease Prevention and Control Technical Report Hepatitis B and C in the EU neighborhood: prevalence, burden of disease and screening policies. Sep, 2010. [Accessed April 14, 2014]. Available at: http://www.ecdc.europa.eu/en/publications/Publications/TER_100914_Hep_B_....
-
- Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362(9401):2095–2100. - PubMed
-
- Gane EJ, Portmann BC, Naoumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334(13):815–820. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

