Lycopene Inhibits Urotensin-II-Induced Cardiomyocyte Hypertrophy in Neonatal Rat Cardiomyocytes
- PMID: 24971153
- PMCID: PMC4058208
- DOI: 10.1155/2014/724670
Lycopene Inhibits Urotensin-II-Induced Cardiomyocyte Hypertrophy in Neonatal Rat Cardiomyocytes
Abstract
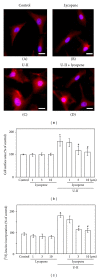
This study investigated how lycopene affected urotensin-II- (U-II-) induced cardiomyocyte hypertrophy and the possible implicated mechanisms. Neonatal rat cardiomyocytes were exposed to U-II (1 nM) either exclusively or following 6 h of lycopene pretreatment (1-10 μ M). The lycopene (3-10 μ M) pretreatment significantly inhibited the U-II-induced cardiomyocyte hypertrophy, decreased the production of U-II-induced reactive oxygen species (ROS), and reduced the level of NAD(P)H oxidase-4 expression. Lycopene further inhibited the U-II-induced phosphorylation of the redox-sensitive extracellular signal-regulated kinases. Moreover, lycopene treatment prevented the increase in the phosphorylation of serine-threonine kinase Akt and glycogen synthase kinase-3beta (GSK-3 β ) caused by U-II without affecting the protein levels of the phosphatase and tensin homolog deleted on chromosome 10 (PTEN). However, lycopene increased the PTEN activity level, suggesting that lycopene prevents ROS-induced PTEN inactivation. These findings imply that lycopene yields antihypertrophic effects that can prevent the activation of the Akt/GSK-3 β hypertrophic pathway by modulating PTEN inactivation through U-II treatment. Thus, the data indicate that lycopene prevented U-II-induced cardiomyocyte hypertrophy through a mechanism involving the inhibition of redox signaling. These findings provide novel data regarding the molecular mechanisms by which lycopene regulates cardiomyocyte hypertrophy.
Figures




References
-
- Swynghedauw B. Remodeling of the heart in chronic pressure overload. Basic Research in Cardiology. 1991;86:99–105. - PubMed
-
- Wang Y. Signal transduction in cardiac hypertrophy—dissecting compensatory versus pathological pathways utilizing a transgenic approach. Current Opinion in Pharmacology. 2001;1(2):134–140. - PubMed
-
- Ames RS, Sarau HM, Chambers JK, et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401(6750):p. 282, p. 286. - PubMed
-
- Ross B, McKendy K, Giaid A. Role of urotensin II in health and disease. American Journal of Physiology, Regulatory Integrative and Comparative Physiology. 2010;298(5):R1156–R1172. - PubMed
-
- Liu J-C, Chen C-H, Chen J-J, Cheng T-H. Urotensin II induces rat cardiomyocyte hypertrophy via the transient oxidization of Src homology 2-containing tyrosine phosphatase and transactivation of epidermal growth factor receptor. Molecular Pharmacology. 2009;76(6):1186–1195. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

