MHC Class I Presented T Cell Epitopes as Potential Antigens for Therapeutic Vaccine against HBV Chronic Infection
- PMID: 24971174
- PMCID: PMC4058288
- DOI: 10.1155/2014/860562
MHC Class I Presented T Cell Epitopes as Potential Antigens for Therapeutic Vaccine against HBV Chronic Infection
Abstract
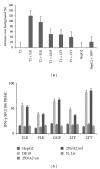
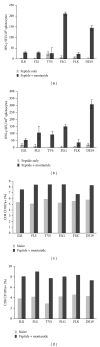
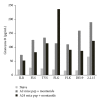
Approximately 370 million people worldwide are chronically infected with hepatitis B virus (HBV). Despite the success of the prophylactic HBV vaccine, no therapeutic vaccine or other immunotherapy modality is available for treatment of chronically infected individuals. Clearance of HBV depends on robust, sustained CD8(+) T activity; however, the limited numbers of therapeutic vaccines tested have not induced such a response. Most of these vaccines have relied on peptide prediction algorithms to identify MHC-I epitopes or characterization of T cell responses during acute infection. Here, we took an immunoproteomic approach to characterize MHC-I restricted epitopes from cells chronically infected with HBV and therefore more likely to represent the true targets of CD8(+) T cells during chronic infection. In this study, we identified eight novel MHC-I restricted epitopes derived from a broad range of HBV proteins that were capable of activating CD8(+) T cells. Furthermore, five of the eight epitopes were able to bind HLA-A2 and A24 alleles and activated HBV specific T cell responses. These epitopes also have potential as new tools to characterize T cell immunity in chronic HBV infection and may serve as candidate antigens for a therapeutic vaccine against HBV infection.
Figures



References
-
- Lok ASF, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34(6):1225–1241. - PubMed
-
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature Reviews Immunology. 2005;5(3):215–229. - PubMed
-
- Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. Journal of General Virology. 2006;87, part 6:1439–1449. - PubMed
-
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiologic Reviews. 2006;28(1):112–125. - PubMed
-
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, arid current and emerging prevention and control measures. Journal of Viral Hepatitis. 2004;11(2):97–107. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

