Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro
- PMID: 24971310
- PMCID: PMC4058294
- DOI: 10.1155/2014/109389
Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro
Abstract
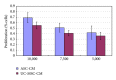
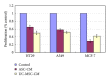
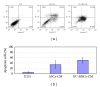
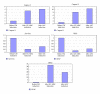
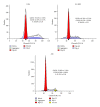
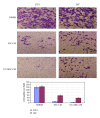
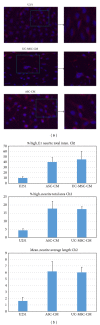
Human mesenchymal stem cells (MSCs) have an intrinsic property for homing towards tumor sites and can be used as tumor-tropic vectors for tumor therapy. But very limited studies investigated the antitumor properties of MSCs themselves. In this study we investigated the antiglioma properties of two easily accessible MSCs, namely, human adipose tissue-derived mesenchymal stem cells (ASCs) and umbilical cord-derived mesenchymal stem cells (UC-MSCs). We found (1) MSC conditioned media can significantly inhibit the growth of human U251 glioma cell line; (2) MSC conditioned media can significantly induce apoptosis in human U251 cell line; (3) real-time PCR experiments showed significant upregulation of apoptotic genes of both caspase-3 and caspase-9 and significant downregulation of antiapoptotic genes such as survivin and XIAP after MSC conditioned media induction in U 251 cells; (4) furthermore, MSCs conditioned media culture induced rapid and complete differentiation in U251 cells. These results indicate MSCs can efficiently induce both apoptosis and differentiation in U251 human glioma cell line. Whereas UC-MSCs are more efficient for apoptosis induction than ASCs, their capability of differentiation induction is not distinguishable from each other. Our findings suggest MSCs themselves have favorable antitumor characteristics and should be further explored in future glioma therapy.
Figures


Similar articles
-
Bone marrow mesenchymal stem cells suppress growth and promote the apoptosis of glioma U251 cells through downregulation of the PI3K/AKT signaling pathway.Biomed Pharmacother. 2019 Apr;112:108625. doi: 10.1016/j.biopha.2019.108625. Epub 2019 Feb 20. Biomed Pharmacother. 2019. PMID: 30784920
-
Side-by-side comparison of the biological characteristics of human umbilical cord and adipose tissue-derived mesenchymal stem cells.Biomed Res Int. 2013;2013:438243. doi: 10.1155/2013/438243. Epub 2013 Jul 7. Biomed Res Int. 2013. Retraction in: Biomed Res Int. 2020 Sep 23;2020:3176431. doi: 10.1155/2020/3176431. PMID: 23936800 Free PMC article. Retracted.
-
Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta.Stem Cell Res Ther. 2016 Nov 10;7(1):163. doi: 10.1186/s13287-016-0418-9. Stem Cell Res Ther. 2016. PMID: 27832825 Free PMC article.
-
Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells.Stem Cells Dev. 2012 Sep 20;21(14):2724-52. doi: 10.1089/scd.2011.0722. Epub 2012 May 9. Stem Cells Dev. 2012. PMID: 22468918 Review.
-
Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues.Cell Prolif. 2017 Apr;50(2):e12334. doi: 10.1111/cpr.12334. Epub 2017 Feb 1. Cell Prolif. 2017. PMID: 28144997 Free PMC article. Review.
Cited by
-
Original Research: Adipose-derived stem cells from younger donors, but not aging donors, inspire the host self-healing capability through its secreta.Exp Biol Med (Maywood). 2017 Jan;242(1):68-79. doi: 10.1177/1535370216662363. Epub 2016 Aug 12. Exp Biol Med (Maywood). 2017. PMID: 27521185 Free PMC article.
-
Effect of Placenta-Derived Mesenchymal Stromal Cells Conditioned Media on an LPS-Induced Mouse Model of Preeclampsia.Int J Mol Sci. 2022 Jan 31;23(3):1674. doi: 10.3390/ijms23031674. Int J Mol Sci. 2022. PMID: 35163594 Free PMC article.
-
Concise Review: Adipose-Derived Stem Cells (ASCs) and Adipocyte-Secreted Exosomal microRNA (A-SE-miR) Modulate Cancer Growth and proMote Wound Repair.J Clin Med. 2019 Jun 15;8(6):855. doi: 10.3390/jcm8060855. J Clin Med. 2019. PMID: 31208047 Free PMC article. Review.
-
Secretion of interleukin-6 by bone marrow mesenchymal stem cells promotes metastasis in hepatocellular carcinoma.Biosci Rep. 2017 Jul 21;37(4):BSR20170181. doi: 10.1042/BSR20170181. Print 2017 Aug 31. Biosci Rep. 2017. PMID: 28659496 Free PMC article.
-
Engineered Mesenchymal Stem Cells as an Anti-Cancer Trojan Horse.Stem Cells Dev. 2016 Oct;25(20):1513-1531. doi: 10.1089/scd.2016.0120. Epub 2016 Sep 7. Stem Cells Dev. 2016. PMID: 27460260 Free PMC article.
References
-
- Wang Y, Jiang T. Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Letters. 2013;331(2):139–146. - PubMed
-
- Bexell D, Svensson A, Bengzon J. Stem cell-based therapy for malignant glioma. Cancer Treatment Reviews. 2013;39(4):358–365. - PubMed
-
- Rahman M, Hoh B, Kohler N, Dunbar EM, Murad GJ. The future of glioma treatment: stem cells, nanotechnology and personalized medicine. Future Oncology. 2012;8(9):1149–1156. - PubMed
-
- Szczepanek D, Marchel A, Moskała M, Krupa M, Kunert P, Trojanowski T. Efficacy of concomitant and adjuvant temozolomide in glioblastoma treatment. A multicentre randomized study. Neurologia i Neurochirurgia Polska. 2013;47(2):101–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

