Cardioprotective effects of osteopontin-1 during development of murine ischemic cardiomyopathy
- PMID: 24971311
- PMCID: PMC4058102
- DOI: 10.1155/2014/124063
Cardioprotective effects of osteopontin-1 during development of murine ischemic cardiomyopathy
Abstract
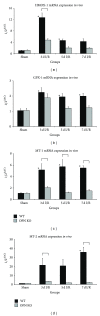
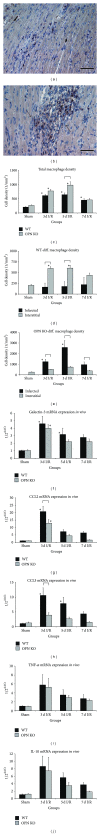
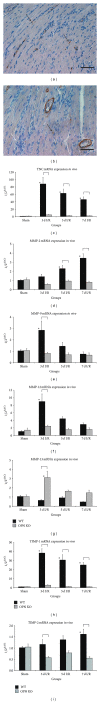
Repetitive brief ischemia and reperfusion (I/R) is associated with ventricular dysfunction in pathogenesis of murine ischemic cardiomyopathy and human hibernating myocardium. We investigated the role of matricellular protein osteopontin-1 (OPN) in murine model of repetitive I/R. One 15-min LAD-occlusion followed by reperfusion was performed daily over 3, 5, and 7 consecutive days in C57/Bl6 wildtype- (WT-) and OPN(-/-)-mice (n = 8/group). After echocardiography hearts were processed for histological and mRNA-studies. Cardiac fibroblasts were isolated, cultured, and stimulated with TGF- β 1. WT-mice showed an early, strong, and cardiomyocyte-specific osteopontin-expression leading to interstitial macrophage infiltration and consecutive fibrosis after 7 days I/R in absence of myocardial infarction. In contrast, OPN(-/-)-mice showed small, nontransmural infarctions after 3 days I/R associated with significantly worse ventricular dysfunction. OPN(-/-)-mice had different expression of myocardial contractile elements and antioxidative mediators and a lower expression of chemokines during I/R. OPN(-/-)-mice showed predominant collagen deposition in macrophage-rich small infarctions. We found lower induction of tenascin-C, MMP-9, MMP-12, and TIMP-1, whereas MMP-13-expression was higher in OPN(-/-)-mice. Cultured OPN(-/-)-myofibroblasts confirmed these findings. In conclusion, osteopontin seems to modulate expression of contractile elements, antioxidative mediators, and inflammatory response and subsequently remodel in order to protect cardiomyocytes in murine ischemic cardiomyopathy.
Figures




References
-
- Kloner RA, Bolli R, Marban E, Reinlib L, Braunwald E. Medical and cellular implications of stunning, hibernation, and preconditioning: an NHLBI workshop. Circulation. 1998;97(18):1848–1867. - PubMed
-
- Frangogiannis NG, Shimoni S, Chang S, et al. Active interstitial remodeling: an important process in the hibernating human myocardium. Journal of the American College of Cardiology. 2002;39(9):1468–1474. - PubMed
-
- Dewald O, Frangogiannis NG, Zoerlein M, et al. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2700–2705. - PMC - PubMed
-
- Frangogiannis NG, Dewald O, Xia Y, et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115(5):584–592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

