Cysteinyl leukotriene receptor-1 antagonists as modulators of innate immune cell function
- PMID: 24971371
- PMCID: PMC4058211
- DOI: 10.1155/2014/608930
Cysteinyl leukotriene receptor-1 antagonists as modulators of innate immune cell function
Abstract
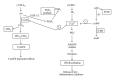
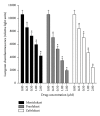
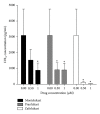
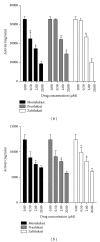
Cysteinyl leukotrienes (cysLTs) are produced predominantly by cells of the innate immune system, especially basophils, eosinophils, mast cells, and monocytes/macrophages. Notwithstanding potent bronchoconstrictor activity, cysLTs are also proinflammatory consequent to their autocrine and paracrine interactions with G-protein-coupled receptors expressed not only on the aforementioned cell types, but also on Th2 lymphocytes, as well as structural cells, and to a lesser extent neutrophils and CD8(+) cells. Recognition of the involvement of cysLTs in the immunopathogenesis of various types of acute and chronic inflammatory disorders, especially bronchial asthma, prompted the development of selective cysLT receptor-1 (cysLTR1) antagonists, specifically montelukast, pranlukast, and zafirlukast. More recently these agents have also been reported to possess secondary anti-inflammatory activities, distinct from cysLTR1 antagonism, which appear to be particularly effective in targeting neutrophils and monocytes/macrophages. Underlying mechanisms include interference with cyclic nucleotide phosphodiesterases, 5'-lipoxygenase, and the proinflammatory transcription factor, nuclear factor kappa B. These and other secondary anti-inflammatory mechanisms of the commonly used cysLTR1 antagonists are the major focus of the current review, which also includes a comparison of the anti-inflammatory effects of montelukast, pranlukast, and zafirlukast on human neutrophils in vitro, as well as an overview of both the current clinical applications of these agents and potential future applications based on preclinical and early clinical studies.
Figures

References
-
- Peters-Golden M, Henderson WR., Jr. Mechanisms of disease: leukotrienes. The New England Journal of Medicine. 2007;357(18):1798–1854. - PubMed
-
- Scott JP, Peters-Golden M. Antileukotriene agents for the treatment of lung disease. American Journal of Respiratory and Critical Care Medicine. 2013;188(5):538–544. - PubMed
-
- Amlani S, Nadarajah T, McIvor RA. Montelukast for the treatment of asthma in the adult population. Expert Opinion on Pharmacotherapy. 2011;12(13):2119–2128. - PubMed
-
- Pacheco Y, Freymond N, Devouassoux G. Impact of montelukast on asthma associated with rhinitis, and other triggers and co-morbidities. Journal of Asthma. 2014;51(1):1–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

