Coupling genetic and chemical microbiome profiling reveals heterogeneity of archaeome and bacteriome in subsurface biofilms that are dominated by the same archaeal species
- PMID: 24971452
- PMCID: PMC4074051
- DOI: 10.1371/journal.pone.0099801
Coupling genetic and chemical microbiome profiling reveals heterogeneity of archaeome and bacteriome in subsurface biofilms that are dominated by the same archaeal species
Abstract
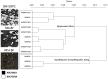
Earth harbors an enormous portion of subsurface microbial life, whose microbiome flux across geographical locations remains mainly unexplored due to difficult access to samples. Here, we investigated the microbiome relatedness of subsurface biofilms of two sulfidic springs in southeast Germany that have similar physical and chemical parameters and are fed by one deep groundwater current. Due to their unique hydrogeological setting these springs provide accessible windows to subsurface biofilms dominated by the same uncultivated archaeal species, called SM1 Euryarchaeon. Comparative analysis of infrared imaging spectra demonstrated great variations in archaeal membrane composition between biofilms of the two springs, suggesting different SM1 euryarchaeal strains of the same species at both aquifer outlets. This strain variation was supported by ultrastructural and metagenomic analyses of the archaeal biofilms, which included intergenic spacer region sequencing of the rRNA gene operon. At 16S rRNA gene level, PhyloChip G3 DNA microarray detected similar biofilm communities for archaea, but site-specific communities for bacteria. Both biofilms showed an enrichment of different deltaproteobacterial operational taxonomic units, whose families were, however, congruent as were their lipid spectra. Consequently, the function of the major proportion of the bacteriome appeared to be conserved across the geographic locations studied, which was confirmed by dsrB-directed quantitative PCR. Consequently, microbiome differences of these subsurface biofilms exist at subtle nuances for archaea (strain level variation) and at higher taxonomic levels for predominant bacteria without a substantial perturbation in bacteriome function. The results of this communication provide deep insight into the dynamics of subsurface microbial life and warrant its future investigation with regard to metabolic and genomic analyses.
Conflict of interest statement
Figures




References
-
- Ulrich GA, Martino D, Burger K, Routh J, Grossman EL, et al. (1998) Sulfur Cycling in the Terrestrial Subsurface: Commensal Interactions, Spatial Scales, and Microbial Heterogeneity. Microb Ecol 36: 141–151. - PubMed
-
- Wrighton KC, Thomas BC, Sharon I, Miller CS, Castelle CJ, et al. (2012) Fermentation, hydrogen, and sulfur metabolism in multiple uncultivated bacterial phyla. Science 337: 1661–1665. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

