Human frataxin activates Fe-S cluster biosynthesis by facilitating sulfur transfer chemistry
- PMID: 24971490
- PMCID: PMC4215901
- DOI: 10.1021/bi500532e
Human frataxin activates Fe-S cluster biosynthesis by facilitating sulfur transfer chemistry
Abstract
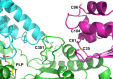
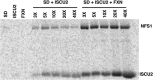
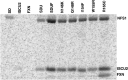
Iron-sulfur clusters are ubiquitous protein cofactors with critical cellular functions. The mitochondrial Fe-S assembly complex, which consists of the cysteine desulfurase NFS1 and its accessory protein (ISD11), the Fe-S assembly protein (ISCU2), and frataxin (FXN), converts substrates l-cysteine, ferrous iron, and electrons into Fe-S clusters. The physiological function of FXN has received a tremendous amount of attention since the discovery that its loss is directly linked to the neurodegenerative disease Friedreich's ataxia. Previous in vitro results revealed a role for human FXN in activating the cysteine desulfurase and Fe-S cluster biosynthesis activities of the Fe-S assembly complex. Here we present radiolabeling experiments that indicate FXN accelerates the accumulation of sulfur on ISCU2 and that the resulting persulfide species is viable in the subsequent synthesis of Fe-S clusters. Additional mutagenesis, enzyme kinetic, UV-visible, and circular dichroism spectroscopic studies suggest conserved ISCU2 residue C104 is critical for FXN activation, whereas C35, C61, and C104 are all essential for Fe-S cluster formation on the assembly complex. These results cannot be fully explained by the hypothesis that FXN functions as an iron donor for Fe-S cluster biosynthesis, and further support an allosteric regulator role for FXN. Together, these results lead to an activation model in which FXN accelerates persulfide formation on NFS1 and favors a helix-to-coil interconversion on ISCU2 that facilitates the transfer of sulfur from NFS1 to ISCU2 as an initial step in Fe-S cluster biosynthesis.
Figures

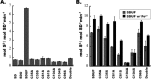


Similar articles
-
Mechanism of activation of the human cysteine desulfurase complex by frataxin.Proc Natl Acad Sci U S A. 2019 Sep 24;116(39):19421-19430. doi: 10.1073/pnas.1909535116. Epub 2019 Sep 11. Proc Natl Acad Sci U S A. 2019. PMID: 31511419 Free PMC article.
-
Mechanism of frataxin "bypass" in human iron-sulfur cluster biosynthesis with implications for Friedreich's ataxia.J Biol Chem. 2019 Jun 7;294(23):9276-9284. doi: 10.1074/jbc.RA119.007716. Epub 2019 Apr 11. J Biol Chem. 2019. PMID: 30975898 Free PMC article.
-
Effector role reversal during evolution: the case of frataxin in Fe-S cluster biosynthesis.Biochemistry. 2012 Mar 27;51(12):2506-14. doi: 10.1021/bi201628j. Epub 2012 Mar 15. Biochemistry. 2012. PMID: 22352884 Free PMC article.
-
Molecular Details of the Frataxin-Scaffold Interaction during Mitochondrial Fe-S Cluster Assembly.Int J Mol Sci. 2021 Jun 2;22(11):6006. doi: 10.3390/ijms22116006. Int J Mol Sci. 2021. PMID: 34199378 Free PMC article. Review.
-
Recent Advances in the Elucidation of Frataxin Biochemical Function Open Novel Perspectives for the Treatment of Friedreich's Ataxia.Front Neurosci. 2022 Mar 2;16:838335. doi: 10.3389/fnins.2022.838335. eCollection 2022. Front Neurosci. 2022. PMID: 35310092 Free PMC article. Review.
Cited by
-
Frataxin deficiency promotes endothelial senescence in pulmonary hypertension.J Clin Invest. 2021 Jun 1;131(11):e136459. doi: 10.1172/JCI136459. J Clin Invest. 2021. PMID: 33905372 Free PMC article.
-
PMPCA mutations cause abnormal mitochondrial protein processing in patients with non-progressive cerebellar ataxia.Brain. 2015 Jun;138(Pt 6):1505-17. doi: 10.1093/brain/awv057. Epub 2015 Mar 25. Brain. 2015. PMID: 25808372 Free PMC article.
-
N-terminal tyrosine of ISCU2 triggers [2Fe-2S] cluster synthesis by ISCU2 dimerization.Nat Commun. 2021 Nov 25;12(1):6902. doi: 10.1038/s41467-021-27122-w. Nat Commun. 2021. PMID: 34824239 Free PMC article.
-
ISCU(M108I) and ISCU(D39V) Differ from Wild-Type ISCU in Their Failure To Form Cysteine Desulfurase Complexes Containing Both Frataxin and Ferredoxin.Biochemistry. 2018 Mar 6;57(9):1491-1500. doi: 10.1021/acs.biochem.7b01234. Epub 2018 Feb 14. Biochemistry. 2018. PMID: 29406711 Free PMC article.
-
Down the Iron Path: Mitochondrial Iron Homeostasis and Beyond.Cells. 2021 Aug 25;10(9):2198. doi: 10.3390/cells10092198. Cells. 2021. PMID: 34571846 Free PMC article. Review.
References
-
- Kessler D. (2006) Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes. FEMS Microbiol. Rev. 30, 825–840. - PubMed
-
- Mueller E. G. (2006) Trafficking in persulfides: Delivering sulfur in biosynthetic pathways. Nat. Chem. Biol. 2, 185–194. - PubMed
-
- Zheng L.; White R. H.; Cash V. L.; Dean D. R. (1994) Mechanism for the desulfurization of l-cysteine catalyzed by the nifS gene product. Biochemistry 33, 4714–4720. - PubMed
-
- Behshad E.; Parkin S. E.; Bollinger J. M. (2004) Mechanism of cysteine desulfurase Slr0387 from Synechocystis sp. PCC 6803: Kinetic analysis of cleavage of the persulfide intermediate by chemical reductants. Biochemistry 43, 12220–12226. - PubMed
-
- Cupp-Vickery J.; Urbina H. D.; Vickery L. (2003) Crystal structure of IscS, a cysteine desulfurase from Escherichia coli. J. Mol. Biol. 330, 1049–1059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

