MutLα heterodimers modify the molecular phenotype of Friedreich ataxia
- PMID: 24971578
- PMCID: PMC4074104
- DOI: 10.1371/journal.pone.0100523
MutLα heterodimers modify the molecular phenotype of Friedreich ataxia
Abstract
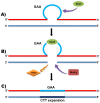
Background: Friedreich ataxia (FRDA), the most common autosomal recessive ataxia disorder, is caused by a dynamic GAA repeat expansion mutation within intron 1 of FXN gene, resulting in down-regulation of frataxin expression. Studies of cell and mouse models have revealed a role for the mismatch repair (MMR) MutS-heterodimer complexes and the PMS2 component of the MutLα complex in the dynamics of intergenerational and somatic GAA repeat expansions: MSH2, MSH3 and MSH6 promote GAA repeat expansions, while PMS2 inhibits GAA repeat expansions.
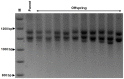
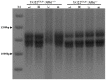
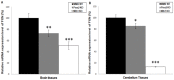
Methodology/principal findings: To determine the potential role of the other component of the MutLα complex, MLH1, in GAA repeat instability in FRDA, we have analyzed intergenerational and somatic GAA repeat expansions from FXN transgenic mice that have been crossed with Mlh1 deficient mice. We find that loss of Mlh1 activity reduces both intergenerational and somatic GAA repeat expansions. However, we also find that loss of either Mlh1 or Pms2 reduces FXN transcription, suggesting different mechanisms of action for Mlh1 and Pms2 on GAA repeat expansion dynamics and regulation of FXN transcription.
Conclusions/significance: Both MutLα components, PMS2 and MLH1, have now been shown to modify the molecular phenotype of FRDA. We propose that upregulation of MLH1 or PMS2 could be potential FRDA therapeutic approaches to increase FXN transcription.
Conflict of interest statement
Figures



Similar articles
-
Replication dependent and independent mechanisms of GAA repeat instability.DNA Repair (Amst). 2022 Oct;118:103385. doi: 10.1016/j.dnarep.2022.103385. Epub 2022 Aug 3. DNA Repair (Amst). 2022. PMID: 35952488 Free PMC article. Review.
-
The mismatch repair system protects against intergenerational GAA repeat instability in a Friedreich ataxia mouse model.Neurobiol Dis. 2012 Apr;46(1):165-71. doi: 10.1016/j.nbd.2012.01.002. Epub 2012 Jan 20. Neurobiol Dis. 2012. PMID: 22289650 Free PMC article.
-
Pms2 suppresses large expansions of the (GAA·TTC)n sequence in neuronal tissues.PLoS One. 2012;7(10):e47085. doi: 10.1371/journal.pone.0047085. Epub 2012 Oct 11. PLoS One. 2012. PMID: 23071719 Free PMC article.
-
Differing patterns of genetic instability in mice deficient in the mismatch repair genes Pms2, Mlh1, Msh2, Msh3 and Msh6.Carcinogenesis. 2006 Dec;27(12):2402-8. doi: 10.1093/carcin/bgl079. Epub 2006 May 25. Carcinogenesis. 2006. PMID: 16728433 Free PMC article.
-
Beyond loss of frataxin: the complex molecular pathology of Friedreich ataxia.Discov Med. 2014 Jan;17(91):25-35. Discov Med. 2014. PMID: 24411698 Review.
Cited by
-
Replication dependent and independent mechanisms of GAA repeat instability.DNA Repair (Amst). 2022 Oct;118:103385. doi: 10.1016/j.dnarep.2022.103385. Epub 2022 Aug 3. DNA Repair (Amst). 2022. PMID: 35952488 Free PMC article. Review.
-
On the wrong DNA track: Molecular mechanisms of repeat-mediated genome instability.J Biol Chem. 2020 Mar 27;295(13):4134-4170. doi: 10.1074/jbc.REV119.007678. Epub 2020 Feb 14. J Biol Chem. 2020. PMID: 32060097 Free PMC article. Review.
-
A Variation in FGF14 Is Associated with Downbeat Nystagmus in a Genome-Wide Association Study.Cerebellum. 2020 Jun;19(3):348-357. doi: 10.1007/s12311-020-01113-x. Cerebellum. 2020. PMID: 32157568 Free PMC article.
-
Triplex H-DNA structure: the long and winding road from the discovery to its role in human disease.NAR Mol Med. 2024 Dec 5;1(4):ugae024. doi: 10.1093/narmme/ugae024. eCollection 2024 Oct. NAR Mol Med. 2024. PMID: 39723156 Free PMC article.
-
Large-scale contractions of Friedreich's ataxia GAA repeats in yeast occur during DNA replication due to their triplex-forming ability.Proc Natl Acad Sci U S A. 2020 Jan 21;117(3):1628-1637. doi: 10.1073/pnas.1913416117. Epub 2020 Jan 7. Proc Natl Acad Sci U S A. 2020. PMID: 31911468 Free PMC article.
References
-
- Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, et al. (1996) Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271: 1423–1427. - PubMed
-
- Saveliev A, Everett C, Sharpe T, Webster Z, Festenstein R (2003) DNA triplet repeats mediate heterochromatin-protein-1-sensitive variegated gene silencing. Nature 422: 909–913. - PubMed
-
- Wells RD (2008) DNA triplexes and Friedreich ataxia. FASEB J 22: 1625–1634. - PubMed
-
- Campuzano V, Montermini L, Lutz Y, Cova L, Hindelang C, et al. (1997) Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum Mol Genet 6: 1771–1780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

