Maternally acquired genotoxic Escherichia coli alters offspring's intestinal homeostasis
- PMID: 24971581
- PMCID: PMC4153768
- DOI: 10.4161/gmic.28932
Maternally acquired genotoxic Escherichia coli alters offspring's intestinal homeostasis
Abstract
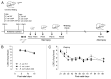
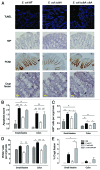
The neonatal gut is rapidly colonized by a newly dominant group of commensal Escherichia coli strains among which a large proportion produces a genotoxin called colibactin. In order to analyze the short- and long-term effects resulting from such evolution, we developed a rat model mimicking the natural transmission of E. coli from mothers to neonates. Genotoxic and non-genotoxic E. coli strains were equally transmitted to the offspring and stably colonized the gut across generations. DNA damage was only detected in neonates colonized with genotoxic E. coli strains. Signs of genotoxic stress such as anaphase bridges, higher occurrence of crypt fission and accelerated renewal of the mature epithelium were detected at adulthood. In addition, we observed alterations of secretory cell populations and gut epithelial barrier. Our findings illustrate how critical is the genotype of E. coli strains acquired at birth for gut homeostasis at adulthood.
Keywords: Escherichia coli; colibactin; epithelial differentiation; epithelial proliferation; genotoxicity; gut; intestinal barrier; neonate.
Figures




Similar articles
-
Oral tolerance failure upon neonatal gut colonization with Escherichia coli producing the genotoxin colibactin.Infect Immun. 2015 Jun;83(6):2420-9. doi: 10.1128/IAI.00064-15. Epub 2015 Mar 30. Infect Immun. 2015. PMID: 25824839 Free PMC article.
-
The Food Contaminant Deoxynivalenol Exacerbates the Genotoxicity of Gut Microbiota.mBio. 2017 Mar 14;8(2):e00007-17. doi: 10.1128/mBio.00007-17. mBio. 2017. PMID: 28292979 Free PMC article.
-
The Genotoxin Colibactin Shapes Gut Microbiota in Mice.mSphere. 2020 Jul 1;5(4):e00589-20. doi: 10.1128/mSphere.00589-20. mSphere. 2020. PMID: 32611705 Free PMC article.
-
Early settlers: which E. coli strains do you not want at birth?Am J Physiol Gastrointest Liver Physiol. 2016 Jul 1;311(1):G123-9. doi: 10.1152/ajpgi.00091.2016. Epub 2016 Jun 10. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27288422 Review.
-
The Enterobacterial Genotoxins: Cytolethal Distending Toxin and Colibactin.EcoSal Plus. 2016 Jul;7(1):10.1128/ecosalplus.ESP-0008-2016. doi: 10.1128/ecosalplus.ESP-0008-2016. EcoSal Plus. 2016. PMID: 27419387 Free PMC article. Review.
Cited by
-
Genome-Wide Identification by Transposon Insertion Sequencing of Escherichia coli K1 Genes Essential for In Vitro Growth, Gastrointestinal Colonizing Capacity, and Survival in Serum.J Bacteriol. 2018 Mar 12;200(7):e00698-17. doi: 10.1128/JB.00698-17. Print 2018 Apr 1. J Bacteriol. 2018. PMID: 29339415 Free PMC article.
-
Robust performance of a live bacterial therapeutic chassis lacking the colibactin gene cluster.PLoS One. 2023 Feb 2;18(2):e0280499. doi: 10.1371/journal.pone.0280499. eCollection 2023. PLoS One. 2023. PMID: 36730255 Free PMC article.
-
Microbial imbalance and intestinal pathologies: connections and contributions.Dis Model Mech. 2014 Oct;7(10):1131-42. doi: 10.1242/dmm.016428. Dis Model Mech. 2014. PMID: 25256712 Free PMC article. Review.
-
The synthesis of the novel Escherichia coli toxin-colibactin and its mechanisms of tumorigenesis of colorectal cancer.Front Microbiol. 2024 Dec 18;15:1501973. doi: 10.3389/fmicb.2024.1501973. eCollection 2024. Front Microbiol. 2024. PMID: 39744397 Free PMC article. Review.
-
Klebsiella oxytoca enterotoxins tilimycin and tilivalline have distinct host DNA-damaging and microtubule-stabilizing activities.Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3774-3783. doi: 10.1073/pnas.1819154116. Epub 2019 Feb 11. Proc Natl Acad Sci U S A. 2019. PMID: 30808763 Free PMC article.
References
-
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
