Resveratrol protects vascular smooth muscle cells against high glucose-induced oxidative stress and cell proliferation in vitro
- PMID: 24971582
- PMCID: PMC4095779
- DOI: 10.12659/MSMBR.890858
Resveratrol protects vascular smooth muscle cells against high glucose-induced oxidative stress and cell proliferation in vitro
Abstract
Background: Resveratrol exhibits beneficial effects against numerous degenerative diseases at different stages of pathogenesis. This study investigated potential mechanisms and resveratrol effects on high glucose (HG)-induced oxidative stress (30 mM D-glucose, 30 min) and cell proliferation (30 mM D-glucose, 24 h) in vascular smooth muscle cells (VSMCs).
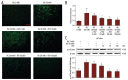
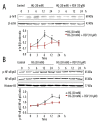
Material/methods: Intracellular reactive oxygen species (ROS) generation was detected by 2',7'-dichlorofluorescein diacetate (DCFH-DA). Total antioxidant capacity (TAC), malonyldialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) were measured to evaluate oxidative stress. VSMC proliferation was measured by CCK-8 assays and through propidium iodide-based cell cycle analysis. Expression of NAD(P)H oxidase, proliferation proteins, and cell signalling were assessed by immunoblot analysis.
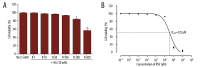
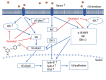
Results: Co-treatment of primary cultures of VSMCs with 1-100 μM resveratrol decreased HG-induced ROS overproduction (P<0.05). Resveratrol also abolished HG-induced phosphorylation of oxidase subunit p47 phox and reduced HG-induced cyclin D1, cyclin E, and PCNA expression in a concentration-dependent manner. Furthermore, resveratrol (10 μM) attenuated HG-induced phosphorylation of Akt, p38 mitogen-activated protein kinase (MAPK), ERK 1/2, and JNK1/2 without affecting total levels. HG stimulation enhanced downstream IκB-α phosphorylation and NF-κB activity, and resveratrol repressed these effects.
Conclusions: Resveratrol inhibits HG-induced oxidative stress and VSMC proliferation by suppressing ROS generation, NADPH oxidase, Akt phosphorylation, p38 MAPK/JNK/ERK phosphorylation, and IκB-α and NF-κB activities.
Figures



Similar articles
-
Resveratrol attenuates hyperproliferation of vascular smooth muscle cells from spontaneously hypertensive rats: Role of ROS and ROS-mediated cell signaling.Vascul Pharmacol. 2018 Feb;101:48-56. doi: 10.1016/j.vph.2017.12.064. Epub 2017 Dec 19. Vascul Pharmacol. 2018. PMID: 29277292
-
High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol.Int J Biochem Cell Biol. 2012 Apr;44(4):629-38. doi: 10.1016/j.biocel.2012.01.001. Epub 2012 Jan 9. Int J Biochem Cell Biol. 2012. PMID: 22245600
-
Resveratrol blocks Akt activation in angiotensin II- or EGF-stimulated vascular smooth muscle cells in a redox-independent manner.Cardiovasc Res. 2011 Apr 1;90(1):140-7. doi: 10.1093/cvr/cvq355. Epub 2010 Nov 10. Cardiovasc Res. 2011. PMID: 21071431 Free PMC article.
-
Synergistic proliferation induced by insulin and glycated serum albumin in rat vascular smooth muscle cells.Sheng Li Xue Bao. 2007 Feb 25;59(1):1-7. Sheng Li Xue Bao. 2007. PMID: 17294035 Review.
-
Human Vascular Smooth Muscle Function and Oxidative Stress Induced by NADPH Oxidase with the Clinical Implications.Cells. 2021 Jul 31;10(8):1947. doi: 10.3390/cells10081947. Cells. 2021. PMID: 34440716 Free PMC article. Review.
Cited by
-
Programming apoptosis and autophagy with novel approaches for diabetes mellitus.Curr Neurovasc Res. 2015;12(2):173-88. doi: 10.2174/1567202612666150305110929. Curr Neurovasc Res. 2015. PMID: 25742566 Free PMC article. Review.
-
Therapeutic Effects of Resveratrol Liposome on Muscle Injury in Rats.Med Sci Monit. 2019 Apr 2;25:2377-2385. doi: 10.12659/MSM.913409. Med Sci Monit. 2019. Retraction in: Med Sci Monit. 2022 Oct 19;28:e938688. doi: 10.12659/MSM.938688. PMID: 30936416 Free PMC article. Retracted.
-
Antibiotics May Trigger Mitochondrial Dysfunction Inducing Psychiatric Disorders.Med Sci Monit. 2017 Jan 7;23:101-106. doi: 10.12659/msm.899478. Med Sci Monit. 2017. PMID: 28063266 Free PMC article. Review.
-
Resveratrol protects against oxidative stress by activating the Keap-1/Nrf2 antioxidant defense system in obese-asthmatic rats.Exp Ther Med. 2018 Dec;16(6):4339-4348. doi: 10.3892/etm.2018.6747. Epub 2018 Sep 17. Exp Ther Med. 2018. PMID: 30542383 Free PMC article.
-
A comparison of resveratrol and other polyphenolic compounds on Notch activation and endothelial cell activity.PLoS One. 2019 Jan 17;14(1):e0210607. doi: 10.1371/journal.pone.0210607. eCollection 2019. PLoS One. 2019. PMID: 30653610 Free PMC article.
References
-
- Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92(22):1037–45. - PubMed
-
- Woodman RJ, Chew GT, Watts GF. Mechanisms, significance and treatment of vascular dysfunction in type 2 diabetes mellitus: focus on lipid-regulating therapy. Drugs. 2005;65(1):31–74. - PubMed
-
- Williams SB, Cusco JA, Roddy MA, et al. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27(3):567–74. - PubMed
-
- Farmer DG, Kennedy S. RAGE, vascular tone and vascular disease. Pharmacol Ther. 2009;124(2):185–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

