Ectosomes: a new mechanism for non-exosomal secretion of tau protein
- PMID: 24971751
- PMCID: PMC4074092
- DOI: 10.1371/journal.pone.0100760
Ectosomes: a new mechanism for non-exosomal secretion of tau protein
Abstract
Tau is a microtubule-associated protein that aggregates in neurodegenerative disorders known as tauopathies. Recently, studies have suggested that Tau may be secreted and play a role in neural network signalling. However, once deregulated, secreted Tau may also participate in the spreading of Tau pathology in hierarchical pathways of neurodegeneration. The mechanisms underlying neuron-to-neuron Tau transfer are still unknown; given the known role of extra-cellular vesicles in cell-to-cell communication, we wondered whether these vesicles could carry secreted Tau. We found, among vesicles, that Tau is predominately secreted in ectosomes, which are plasma membrane-originating vesicles, and when it accumulates, the exosomal pathway is activated.
Conflict of interest statement
Figures
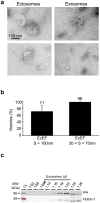
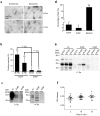
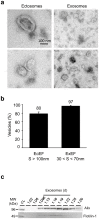
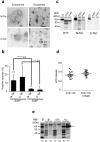

References
-
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33: 95–130. - PubMed
-
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: 239–259. - PubMed
-
- Delacourte A, David JP, Sergeant N, Buee L, Wattez A, et al. (1999) The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology 52: 1158–1165. - PubMed
-
- Verny M, Duyckaerts C, Agid Y, Hauw JJ (1996) The significance of cortical pathology in progressive supranuclear palsy. Clinico-pathological data in 10 cases. Brain 119 (Pt 4): 1123–1136. - PubMed
-
- Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, et al. (2004) Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol 63: 911–918. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

