Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential
- PMID: 24971815
- PMCID: PMC4192010
- DOI: 10.1016/j.steroids.2014.06.012
Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential
Abstract
Estrogen receptors alpha (ERα) and beta (ERβ) are nuclear transcription factors that are involved in the regulation of many complex physiological processes in humans. Modulation of these receptors by prospective therapeutic agents is currently being considered for prevention and treatment of a wide variety of pathological conditions, such as, cancer, metabolic and cardiovascular diseases, neurodegeneration, inflammation, and osteoporosis. This review provides an overview and update of compounds that have been recently reported as modulators of ERs, with a particular focus on their potential clinical applications.
Keywords: Agonists; Antagonists; Estrogen receptor subtype α; Estrogen receptor subtype β; Modulators; Therapeutic applications.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
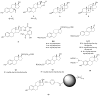
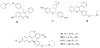
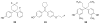
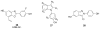

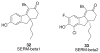
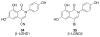

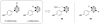
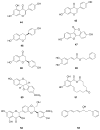
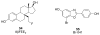
References
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM. Writing Group for the Women’s Health Initiative: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. - PubMed
-
- Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JÅ International Union of Pharmacology. LXIV. Estrogen Receptors. Pharmacol Rev. 2006;58:773–781. - PubMed
-
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JÅ. Estrogen receptors: how do they signal and what are their targets. Physiological Reviews. 2007;87:905–931. - PubMed
-
- Jordan VC. Selective estrogen receptor modulation: a personal perspective. Cancer Res. 2001;61:5683–5687. - PubMed
-
- Brzozowski AM, Pike ACW, Dauter Z, Hubbard RE, Bonn T, Engström O, Öhman L, Greene GL, Gustafsson JÅ, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

