Cep192 controls the balance of centrosome and non-centrosomal microtubules during interphase
- PMID: 24971877
- PMCID: PMC4074188
- DOI: 10.1371/journal.pone.0101001
Cep192 controls the balance of centrosome and non-centrosomal microtubules during interphase
Abstract
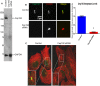
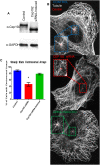
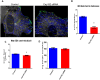
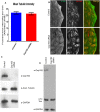
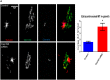
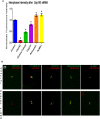
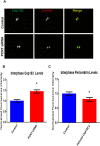
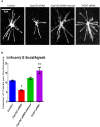
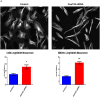
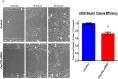
Cep192 is a centrosomal protein that contributes to the formation and function of the mitotic spindle in mammalian cells. Cep192's mitotic activities stem largely from its role in the recruitment to the centrosome of numerous additional proteins such as gamma-tubulin and Pericentrin. Here, we examine Cep192's function in interphase cells. Our data indicate that, as in mitosis, Cep192 stimulates the nucleation of centrosomal microtubules thereby regulating the morphology of interphase microtubule arrays. Interestingly, however, cells lacking Cep192 remain capable of generating normal levels of MTs as the loss of centrosomal microtubules is augmented by MT nucleation from other sites, most notably the Golgi apparatus. The depletion of Cep192 results in a significant decrease in the level of centrosome-associated gamma-tubulin, likely explaining its impact on centrosome microtubule nucleation. However, in stark contrast to mitosis, Cep192 appears to maintain an antagonistic relationship with Pericentrin at interphase centrosomes. Interphase cells depleted of Cep192 display significantly higher levels of centrosome-associated Pericentrin while overexpression of Cep192 reduces the levels of centrosomal Pericentrin. Conversely, depletion of Pericentrin results in elevated levels of centrosomal Cep192 and enhances microtubule nucleation at centrosomes, at least during interphase. Finally, we show that depletion of Cep192 negatively impacts cell motility and alters normal cell polarization. Our current working hypothesis is that the microtubule nucleating capacity of the interphase centrosome is determined by an antagonistic balance of Cep192, which promotes nucleation, and Pericentrin, which inhibits nucleation. This in turn determines the relative abundance of centrosomal and non-centrosomal microtubules that tune cell movement and shape.
Conflict of interest statement
Figures
Similar articles
-
The dual role of the centrosome in organizing the microtubule network in interphase.EMBO Rep. 2018 Nov;19(11):e45942. doi: 10.15252/embr.201845942. Epub 2018 Sep 17. EMBO Rep. 2018. PMID: 30224411 Free PMC article.
-
Human Cep192 is required for mitotic centrosome and spindle assembly.Curr Biol. 2007 Nov 20;17(22):1960-6. doi: 10.1016/j.cub.2007.10.019. Epub 2007 Nov 1. Curr Biol. 2007. PMID: 17980596
-
Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry.Mol Biol Cell. 2004 Aug;15(8):3642-57. doi: 10.1091/mbc.e03-11-0796. Epub 2004 May 14. Mol Biol Cell. 2004. PMID: 15146056 Free PMC article.
-
Cep192 and the generation of the mitotic spindle.Cell Cycle. 2008 Jun 1;7(11):1507-10. doi: 10.4161/cc.7.11.5957. Epub 2008 Mar 18. Cell Cycle. 2008. PMID: 18469523 Review.
-
Centrosome function: sometimes less is more.Traffic. 2009 May;10(5):472-81. doi: 10.1111/j.1600-0854.2009.00880.x. Epub 2009 Jan 24. Traffic. 2009. PMID: 19192251 Review.
Cited by
-
Nonrandom γ-TuNA-dependent spatial pattern of microtubule nucleation at the Golgi.Mol Biol Cell. 2017 Nov 7;28(23):3181-3192. doi: 10.1091/mbc.E17-06-0425. Epub 2017 Sep 20. Mol Biol Cell. 2017. PMID: 28931596 Free PMC article.
-
Atypical function of a centrosomal module in WNT signalling drives contextual cancer cell motility.Nat Commun. 2019 May 29;10(1):2356. doi: 10.1038/s41467-019-10241-w. Nat Commun. 2019. PMID: 31142743 Free PMC article.
-
Brain multi-omic Mendelian randomisation to identify novel drug targets for gliomagenesis.Hum Mol Genet. 2025 Jan 29;34(2):178-192. doi: 10.1093/hmg/ddae168. Hum Mol Genet. 2025. PMID: 39565278 Free PMC article.
-
Human centrosome organization and function in interphase and mitosis.Semin Cell Dev Biol. 2021 Sep;117:30-41. doi: 10.1016/j.semcdb.2021.03.020. Epub 2021 Apr 6. Semin Cell Dev Biol. 2021. PMID: 33836946 Free PMC article. Review.
-
CEP192 is a novel prognostic marker and correlates with the immune microenvironment in hepatocellular carcinoma.Front Immunol. 2022 Sep 27;13:950884. doi: 10.3389/fimmu.2022.950884. eCollection 2022. Front Immunol. 2022. PMID: 36238304 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

