The tumor suppressor role of miR-124 in osteosarcoma
- PMID: 24971902
- PMCID: PMC4074038
- DOI: 10.1371/journal.pone.0091566
The tumor suppressor role of miR-124 in osteosarcoma
Retraction in
-
Retraction: The Tumor Suppressor Role of miR-124 in Osteosarcoma.PLoS One. 2019 Mar 13;14(3):e0214018. doi: 10.1371/journal.pone.0214018. eCollection 2019. PLoS One. 2019. PMID: 30865727 Free PMC article. No abstract available.
Abstract
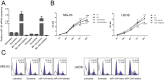
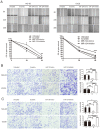
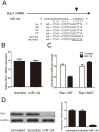
MicroRNAs have crucial roles in development and progression of human cancers, including osteosarcoma. Recent studies have shown that miR-124 was down-regulated in many cancers; however, the role of miR-124 in osteosarcoma development is unknown. In this study, we demonstrate that expression of miR-124 is significantly downregulated in osteosarcoma tissues and cell lines, compared to the adjacent tissues. The expression of miR-124 in the metastases osteosarcoma tissues was lower than that in non- metastases tissues. We identified and confirmed Rac1 as a novel, direct target of miR-124 using prediction algorithms and luciferase reporter gene assays. Overexpression of miR-124 suppressed Rac1 protein expression and attenuated cell proliferation, migration, and invasion and induced apoptosis in MG-63 and U2OS in vitro. Moreover, overexpression of Rac1 in miR-124-transfected osteosarcoma cells effectively rescued the inhibition of cell invasion caused by miR-124. Therefore, our results demonstrate that miR-124 is a tumor suppressor miRNA and suggest that this miRNA could be a potential target for the treatment of osteosarcoma in future.
Conflict of interest statement
Figures


References
-
- Yang J, Zhang W (2013) New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol 25: 398–406. - PubMed
-
- Ottaviani G, Jaffe N (2009) The epidemiology of osteosarcoma. Cancer Treat Res 152: 3–13. - PubMed
-
- Gougelet A, Pissaloux D, Besse A, Perez J, Duc A, et al. (2011) Micro-RNA profiles in osteosarcoma as a predictive tool for ifosfamide response. Int J Cancer 129: 680–690. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

