Possible roles of glutamate transporter EAAT5 in mouse cone depolarizing bipolar cell light responses
- PMID: 24972005
- PMCID: PMC4545533
- DOI: 10.1016/j.visres.2014.06.005
Possible roles of glutamate transporter EAAT5 in mouse cone depolarizing bipolar cell light responses
Abstract
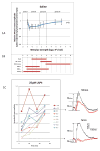
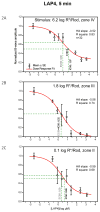
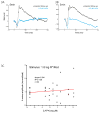
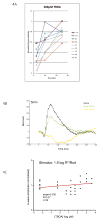
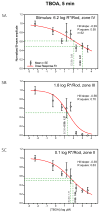
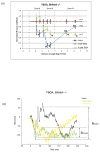
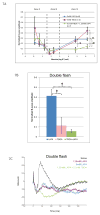
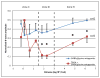
A remarkable feature of neuronal glutamate transporters (EAATs) is their dual functions of classical carriers and ligand-gated chloride (Cl(-)) channels. Cl(-) conductance is rapidly activated by glutamate in subtype EAAT5, which mediates light responses in depolarizing bipolar cells (DBC) in retinae of lower vertebrates. In this study, we examine whether EAAT5 also mediates the DBC light response in mouse. We took advantage of an infrared illuminated micro-injection system, and studied the effects of the EAAT blocker (TBOA) and a glutamate receptor agonist (LAP4) on the mouse electroretinogram (ERG) b-wave responses. Our results showed that TBOA and LAP4 shared similar temporal patterns of inhibition: both inhibited the ERG b-wave shortly after injection and recovered with similar time courses. TBOA inhibited the b-wave completely at mesopic light intensity with an IC50 value about 1 log unit higher than that of LAP4. The inhibitory effects of TBOA and LAP4 were found to be additive in the photopic range. Furthermore, TBOA alone inhibited the b-wave in the cone operative range in knockout mice lacking DBCRs at a low concentration that did not alter synaptic glutamate clearance activity. It also produced a stronger inhibition than that of LAP4 on the cone-driven b-wave measured with a double flash method in wildtype mice. These electrophysiological data suggest a significant role for EAAT5 in mediating cone-driven DBC light responses. Our immunohistochemistry data indicated the presence of postsynaptic EAAT5 on some DBCCs and some DBCRs, providing an anatomical basis for EAAT5's role in DBC light responses.
Keywords: Bipolar cells; EAAT5; Electroretinogram; Glutamate transporter; Immunohistochemistry; Retina.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
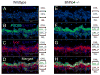
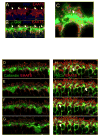
Similar articles
-
EAAT5 Glutamate Transporter-Mediated Inhibition in the Vertebrate Retina.Front Cell Neurosci. 2021 May 6;15:662859. doi: 10.3389/fncel.2021.662859. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34025361 Free PMC article. Review.
-
Pharmacological inhibitions of glutamate transporters EAAT1 and EAAT2 compromise glutamate transport in photoreceptor to ON-bipolar cell synapses.Vision Res. 2014 Oct;103:49-62. doi: 10.1016/j.visres.2014.07.020. Epub 2014 Aug 22. Vision Res. 2014. PMID: 25152321 Free PMC article.
-
The glutamate transporter EAAT5 works as a presynaptic receptor in mouse rod bipolar cells.J Physiol. 2006 Nov 15;577(Pt 1):221-34. doi: 10.1113/jphysiol.2006.118281. Epub 2006 Sep 14. J Physiol. 2006. PMID: 16973698 Free PMC article.
-
Light-evoked glutamate transporter EAAT5 activation coordinates with conventional feedback inhibition to control rod bipolar cell output.J Neurophysiol. 2020 May 1;123(5):1828-1837. doi: 10.1152/jn.00527.2019. Epub 2020 Apr 1. J Neurophysiol. 2020. PMID: 32233906 Free PMC article.
-
Mouse b-wave mutants.Doc Ophthalmol. 2014 Apr;128(2):77-89. doi: 10.1007/s10633-013-9424-8. Epub 2014 Jan 7. Doc Ophthalmol. 2014. PMID: 24395437 Free PMC article. Review.
Cited by
-
EAAT5 Glutamate Transporter-Mediated Inhibition in the Vertebrate Retina.Front Cell Neurosci. 2021 May 6;15:662859. doi: 10.3389/fncel.2021.662859. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34025361 Free PMC article. Review.
-
ON-OFF Interactions in the Retina: Role of Glycine and GABA.Curr Neuropharmacol. 2014 Dec;12(6):509-26. doi: 10.2174/1570159X13999150122165018. Curr Neuropharmacol. 2014. PMID: 25977678 Free PMC article.
-
Improved retinal function in RCS rats after suppressing the over-activation of mGluR5.Sci Rep. 2017 Jun 14;7(1):3546. doi: 10.1038/s41598-017-03702-z. Sci Rep. 2017. PMID: 28615682 Free PMC article.
-
The Regulation of Astrocytic Glutamate Transporters in Health and Neurodegenerative Diseases.Int J Mol Sci. 2020 Dec 17;21(24):9607. doi: 10.3390/ijms21249607. Int J Mol Sci. 2020. PMID: 33348528 Free PMC article. Review.
-
Excitatory Amino Acid Transporter EAAT5 Improves Temporal Resolution in the Retina.eNeuro. 2021 Dec 10;8(6):ENEURO.0406-21.2021. doi: 10.1523/ENEURO.0406-21.2021. Print 2021 Nov-Dec. eNeuro. 2021. PMID: 34772693 Free PMC article.
References
-
- Applebury ML, Antoch MP, et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27(3):513–523. - PubMed
-
- Attwell D. The photoreceptor output synapse. In: Osborne N, Chader G, editors. Progress in Retinal Research. Oxford: Pergamon Press; 1990. p. 9.
-
- Awatramani G, Wang J, et al. Amacrine and ganglion cell contributions to the electroretinogram in amphibian retina. Visual neuroscience. 2001;18(1):147–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

