The use of supported acidic ionic liquids in organic synthesis
- PMID: 24972271
- PMCID: PMC6271805
- DOI: 10.3390/molecules19078840
The use of supported acidic ionic liquids in organic synthesis
Abstract
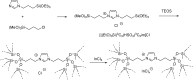
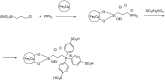
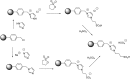
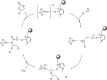
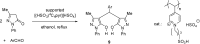
Catalysts obtained by the immobilisation of acidic ionic liquids (ILs) on solid supports offer several advantages compared to the use of catalytically active ILs themselves. Immobilisation may result in an increase in the number of accessible active sites of the catalyst and a reduction of the amount of the IL required. The ionic liquid films on the carrier surfaces provide a homogeneous environment for catalytic reactions but the catalyst appears macroscopically as a dry solid, so it can simply be separated from the reaction mixture. As another advantage, it can easily be applied in a continuous fixed bed reactor. In the present review the main synthetic strategies towards the preparation of supported Lewis acidic and Brønsted acidic ILs are summarised. The most important characterisation methods and structural features of the supported ionic liquids are presented. Their efficiency in catalytic reactions is discussed with special emphasis on their recyclability.
Conflict of interest statement
The author declares no conflict of interest.
Figures

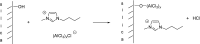




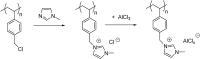
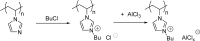
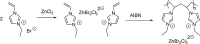




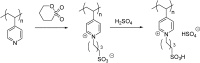

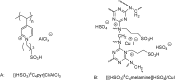
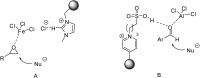


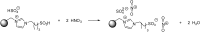
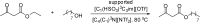
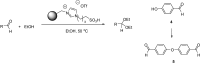

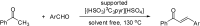






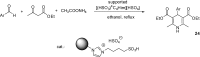

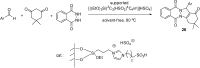
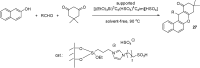

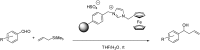
References
-
- Wilson K., Clark J.H. Solid acids and their use as environmentally friendly catalysts in organic synthesis. Pure Appl. Chem. 2000;72:1313–1319.
-
- Hajipour A.R., Rafiee F. Acidic bronsted ionic liquids. Org. Prep. Proc. Int. 2010;42:285–362. doi: 10.1080/00304948.2010.490177. - DOI
-
- Lin I.J.B., Vasam C.S. Metal-containing ionic liquids and ionic liquid crystals based on imidazolium moiety. J. Organomet. Chem. 2005;690:3498–3512. doi: 10.1016/j.jorganchem.2005.03.007. - DOI
-
- Chiappe C., Rajamani S. Structural effects on the physico-chemical and catalytic properties of acidic ionic liquids: An overview. Eur. J. Org. Chem. 2011;28:5517–5539. doi: 10.1002/ejoc.201100432. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

